Application of Deuterium in an M1 Positive Allosteric Modulator Back-Up Program: The Discovery of VU6045422
- PMID: 40132023
- PMCID: PMC12006963
- DOI: 10.1021/acschemneuro.5c00119
Application of Deuterium in an M1 Positive Allosteric Modulator Back-Up Program: The Discovery of VU6045422
Abstract
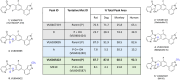
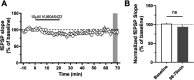
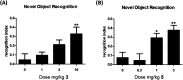
Recently, we disclosed VU0467319, an M1 positive allosteric modulator (PAM) clinical candidate that had successfully completed a phase I single ascending dose clinical trial. Pharmacokinetic assessment revealed that, in humans upon increasing dose, a circulating, inactive metabolite constituted a major portion of the total drug-related area under the curve (AUC). One approach the team employed to reduce inactive metabolite formation in the back-up program was the kinetic isotope effect, replacing the metabolically labile C-H bonds with shorter, more stable C-D bonds. The C-D dipole afforded VU6045422, a more potent M1 PAM (human EC50 = 192 nM, 80% ACh Max) than its proteocongener VU0467319 (human EC50 = 492 nM, 71% ACh Max), and retained the desired profile of minimal M1 agonism. Overall, the profile of VU6045422 supported advancement, as did greater in vitro metabolic stability in both microsomes and hepatocytes than did VU0467319. In both rat and dog in vivo, low doses proved to mirror the in vitro profile; however, at higher doses in 14-day exploratory toxicology studies, the amount of the same undesired metabolite derived from VU6045422 was equivalent to that produced from VU0467319. This unexpected IVIVC result, coupled with less than dose-proportional increases in exposure and no improvement in solubility, led to discontinuation of VU0467319/VU6045422 development.
Keywords: cognition; deuterium; isotope; metabolism; muscarinic acetylcholine receptor subtype 1 (M1); positive allosteric modulator (PAM).
Conflict of interest statement
The authors declare the following competing financial interest(s): The WCNDD and Acadia are actively developing M1 PAMs for clinical development and profit. The M1 PAMs in this paper, while covered by a patent, are no longer under development or being pursued.
Figures


Similar articles
-
Discovery of VU0467319: an M1 Positive Allosteric Modulator Candidate That Advanced into Clinical Trials.ACS Chem Neurosci. 2025 Jan 1;16(1):95-107. doi: 10.1021/acschemneuro.4c00769. Epub 2024 Dec 11. ACS Chem Neurosci. 2025. PMID: 39660766 Free PMC article. Clinical Trial.
-
Discovery of VU6007496: Challenges in the Development of an M1 Positive Allosteric Modulator Backup Candidate.ACS Chem Neurosci. 2024 Sep 18;15(18):3421-3433. doi: 10.1021/acschemneuro.4c00508. Epub 2024 Aug 28. ACS Chem Neurosci. 2024. PMID: 39197083 Free PMC article.
-
Discovery and Optimization of Potent and CNS Penetrant M5-Preferring Positive Allosteric Modulators Derived from a Novel, Chiral N-(Indanyl)piperidine Amide Scaffold.ACS Chem Neurosci. 2018 Jul 18;9(7):1572-1581. doi: 10.1021/acschemneuro.8b00126. Epub 2018 Apr 26. ACS Chem Neurosci. 2018. PMID: 29678111 Free PMC article.
-
M1 receptor positive allosteric modulators discovery approaches.Trends Pharmacol Sci. 2025 Apr;46(4):298-302. doi: 10.1016/j.tips.2025.03.001. Epub 2025 Mar 25. Trends Pharmacol Sci. 2025. PMID: 40133192 Review.
-
Development of allosteric modulators of GPCRs for treatment of CNS disorders.Neurobiol Dis. 2014 Jan;61:55-71. doi: 10.1016/j.nbd.2013.09.013. Epub 2013 Sep 27. Neurobiol Dis. 2014. PMID: 24076101 Free PMC article. Review.
References
-
- Greenlee W.; Clader J.; Asberom T.; McCombie S.; Ford J.; Guzik H.; Kozlowski J.; Li S.; Liu C.; Lowe D.; Vice S.; Zhao H.; Zhou G.; Billard W.; Binch H.; Crosby R.; Duffy R.; Lachowicz J.; Coffin V.; Watkins R.; Ruperto V.; Strader C.; Taylor L.; Cox K. Muscarinic agonists in the treatment of Alzheimer’s disease. Farmaco 2001, 56, 247–250. 10.1016/S0014-827X(01)01102-8. - DOI - PubMed
-
- Fisher A. Muscarinic receptor agonists in Alzheimer’s disease. CNS Drugs 1999, 12, 197–214. 10.2165/00023210-199912030-00004. - DOI
-
- Clader J. W. Recent advances in cholinergic drugs for Alzheimer’s disease. Curr. Opin. Drug Discovery Dev. 1999, 2, 311–320. - PubMed
-
- Melancon B. J.; Tarr J. C.; Panarese J. D.; Wood M. R.; Lindsley C. W. Allosteric modulation of the M1 muscarinic acetylcholine receptor: improving cognition and a potential treatment for schizophrenia and Alzheimer’s disease. Drug Discovery Today 2013, 18 (23–24), 1185–1199. 10.1016/j.drudis.2013.09.005. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

