Flecainide acetate inhalation solution for cardioversion of recent-onset, symptomatic atrial fibrillation: results of the phase 3 RESTORE-1 trial
- PMID: 40132102
- PMCID: PMC11992556
- DOI: 10.1093/europace/euaf064
Flecainide acetate inhalation solution for cardioversion of recent-onset, symptomatic atrial fibrillation: results of the phase 3 RESTORE-1 trial
Abstract
Aims: Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia. New treatments are needed to cardiovert recent-onset paroxysmal AF quickly and safely. RESTORE-1 was a multicentre, randomized, double-blind, placebo-controlled trial of a 120 mg orally inhaled solution of flecainide acetate (FlecIH-103) for cardioversion of symptomatic, recent-onset (≤48 h) paroxysmal AF. The study aim was to evaluate the efficacy and safety of FlecIH-103 administered via oral inhalation.
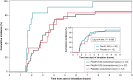
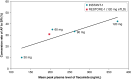
Methods and results: Patients experiencing a recent-onset paroxysmal AF episode were randomized to receive a single dose of FlecIH-103 or placebo delivered over two 3.5 min inhalation periods, while patients were monitored using 12-lead electrocardiograms and Holter. The trial was stopped prematurely after treating 55 patients, due to lower-than-expected conversion rates and plasma levels. Mean age was 59.6 years, 31.5% of patients were female, and 59.2% were having their first AF episode. Conversion rate was 30.8% (95% confidence interval: 14.7-43.8) for the active group (n = 39) and 0.0% for the placebo group (n = 12) (P = 0.04). Median time to conversion was 12.8 min (IQR: 17.2). In the active group, the mean flecainide plasma level was 198 ng/mL (SD: 156), which is ∼50% lower than in the previous studies. The most common adverse events (AEs) were dysgeusia, dyspnoea, and cough. All AEs were short-lasting and of mild or moderate intensity.
Conclusion: Despite early termination of the trial, FlecIH-103 was significantly more effective than placebo in cardioverting AF. Safety data did not show any serious AEs. Further studies of FlecIH-103 are needed to optimize the combination of drug formulation and inhalation delivery platform.
Clinical trial registration: URL: https://clinicaltrials.gov, unique identifier: NCT05039359.
Keywords: Atrial fibrillation; Cardioversion; Flecainide; Oral inhalation.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: M.R. has received consultancy fees from Bayer and InCarda Therapeutics. A.C.W.-S. and D.P. have received consultancy fees from InCarda Therapeutics. Y.T. is a shareholder of DRC B.V. J.L.M. has received fees and honoraria for lectures, education, or scientific advice and institutional fellow grant support from Abbott, AstraZeneca, Biotronik, InCarda, Medtronic, Microport, Milestone Pharmaceuticals, Sanofi, and Zoll. J.N.R. has served as a consultant for Acesion Pharma, Advanced Medical Education, InCarda Therapeutics, and Janssen and holds equity in Celero Systems, Element Science, Infobionic, LuxCath, and NewPace. A.J.C. has received personal fees from Acesion, Anthos, InCarda, Menarini, Milestone, Sanofi, Bayer, Daiichi Sankyo, Pfizer, Abbott, Biosense Webster, Biotronik, Boston Scientific, Medtronic, and Johnson and Johnson. P.R.K. has served as a consultant for Abbvie, InCarda, Milestone, Acesion, Novartis, and HUYA Biopharma. C.D. was an employee of InCarda Therapeutics during study execution. J.M. and L.B. are employees of InCarda Therapeutics. The remaining authors report no conflicts of interest to disclose.
Figures




Comment in
-
Flecainide and atrial fibrillation cardioversion: what solutions at present and in the near future?Europace. 2025 Mar 28;27(4):euaf063. doi: 10.1093/europace/euaf063. Europace. 2025. PMID: 40165417 Free PMC article. No abstract available.
References
-
- Iwasaki Y, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology. Circulation 2011;124:2264–74. - PubMed
-
- Martín A, Coll-Vinent B, Suero C, Fernández-Simón A, Sánchez J, Varona M et al. Benefits of rhythm control and rate control in recent-onset atrial fibrillation: the HERMES-AF study. Acad Emerg Med 2019;26:1034–43. - PubMed
-
- Mohamed MS, Hashem A, Khalouf A, Osama M, Pendela VS, Rai D et al. Delayed vs early cardioversion in patients with paroxysmal atrial fibrillation: a population-based study (2015–2020). Future Cardiol 2023;19:441–52. - PubMed
-
- Khan IA. Oral loading single dose flecainide for pharmacological cardioversion of recent-onset atrial fibrillation. Int J Cardiol 2003;87:121–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

