Evaluation of Microfilaremic Individuals after Mass Drug Treatment with Ivermectin, Diethylcarbamazine, and Albendazole for Lymphatic Filariasis in Papua New Guinea
- PMID: 40132219
- PMCID: PMC12139549
- DOI: 10.4269/ajtmh.24-0382
Evaluation of Microfilaremic Individuals after Mass Drug Treatment with Ivermectin, Diethylcarbamazine, and Albendazole for Lymphatic Filariasis in Papua New Guinea
Abstract
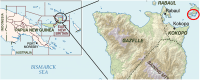
After mass drug administration (MDA) for lymphatic filariasis, which involved a single coadministered dose of ivermectin plus diethylcarbamazine and albendazole (IDA), concerns arose regarding individuals who remained microfilaremic. This situation raised questions about the efficacy of the drugs and whether some individuals had not ingested them. In East New Britain Province, Papua New Guinea (PNG), where 81.7% of the population received IDA, 10 individuals were found to have microfilaremia 12 months after the first round of MDA in an area that had a high baseline of microfilaremia (n = 29 microfilariae [Mf] positive pre-MDA). Of these 10 individuals, 7 reported having taken the IDA medication. When Mf detection was repeated 18 months later, all 10 individuals remained Mf positive. Additionally, three more Mf-positive household members were identified, and they also reported taking the IDA. These Mf-positive individuals were then retreated with IDA under direct observation. At 7 and/or 14 months after retreatment, all initially Mf-positive individuals, except for one, were found to be Mf free. Upon further questioning, it was revealed that all but one individual admitted to not taking the initial MDA. Thus, IDA effectively clears Mf in this region of PNG, and the persistent microfilaremia after MDA is primarily because of individuals failing to take the medications as prescribed.
Conflict of interest statement
Disclosures: The protocol was approved by the Medical Research Advisory Council of Papua New Guinea (MRAC 174 19.14) and the Case Western Reserve University Institutional Review Board (STUDY20191141). Adults ages 18 years old and older provided written informed consent. If they could not read or write, a village member who could read or write served as a witness and cosigned the informed consent form. Children’s participation required the consent of their parents and documented assent for children older than 12 years of age.
Figures
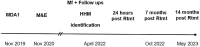
Similar articles
-
Alternative approaches for monitoring and evaluation of lymphatic filariasis following mass drug treatment with ivermectin, diethylcarbamazine and albendazole in East New Britain Province, Papua New Guinea.PLoS Negl Trop Dis. 2025 Jan 27;19(1):e0012128. doi: 10.1371/journal.pntd.0012128. eCollection 2025 Jan. PLoS Negl Trop Dis. 2025. PMID: 39869653 Free PMC article.
-
Safety and efficacy of mass drug administration with a single-dose triple-drug regimen of albendazole + diethylcarbamazine + ivermectin for lymphatic filariasis in Papua New Guinea: An open-label, cluster-randomised trial.PLoS Negl Trop Dis. 2022 Feb 9;16(2):e0010096. doi: 10.1371/journal.pntd.0010096. eCollection 2022 Feb. PLoS Negl Trop Dis. 2022. PMID: 35139070 Free PMC article. Clinical Trial.
-
An open label, block randomized, community study of the safety and efficacy of co-administered ivermectin, diethylcarbamazine plus albendazole vs. diethylcarbamazine plus albendazole for lymphatic filariasis in India.PLoS Negl Trop Dis. 2021 Feb 16;15(2):e0009069. doi: 10.1371/journal.pntd.0009069. eCollection 2021 Feb. PLoS Negl Trop Dis. 2021. PMID: 33591979 Free PMC article. Clinical Trial.
-
Model-based analysis of trial data: microfilaria and worm-productivity loss after diethylcarbamazine-albendazole or ivermectin-albendazole combination therapy against Wuchereria bancrofti.Trop Med Int Health. 2006 May;11(5):718-28. doi: 10.1111/j.1365-3156.2006.01606.x. Trop Med Int Health. 2006. PMID: 16640625 Review.
-
Antifilarial treatment strategies: a systematic review and network meta-analysis.BMC Infect Dis. 2025 May 16;25(1):712. doi: 10.1186/s12879-025-11105-z. BMC Infect Dis. 2025. PMID: 40380307 Free PMC article. Review.
References
-
- WHO, 2022. Global Programme to Eliminate Lymphatic Filariasis: Progress Report, 2021. Weekly Epidemiological Record. Available at: https://www.who.int/publications/i/item/who-wer9741-513-524. Accessed May 22, 2024.
-
- Bjerum CM, Ouattara AF, Aboulaye M, Kouadio O, Marius VK, Andersen BJ, Weil GJ, Koudou BG, King CL, 2020. Efficacy and safety of a single dose of ivermectin, diethylcarbamazine, and albendazole for treatment of lymphatic filariasis in Cote d’Ivoire: An open-label randomized controlled trial. Clin Infect Dis 71: e68–e75. - PMC - PubMed
-
- Thomsen EK. et al., 2016. Efficacy, safety, and pharmacokinetics of coadministered diethylcarbamazine, albendazole, and ivermectin for treatment of Bancroftian filariasis. Clin Infect Dis 62: 334–341. - PubMed
-
- WHO, 2017. Guideline: Alternative Mass Drug Administration Regimens to Eliminate Lymphatic Filariasis. Geneva, Switzerland: World Health Organization. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

