Dihydromyricetin ameliorates lipopolysaccharide‒induced hepatic injury in chickens by activating the Nrf2/Keap1 pathway and regulating mitochondrial dynamics
- PMID: 40132312
- PMCID: PMC11986534
- DOI: 10.1016/j.psj.2025.105034
Dihydromyricetin ameliorates lipopolysaccharide‒induced hepatic injury in chickens by activating the Nrf2/Keap1 pathway and regulating mitochondrial dynamics
Abstract
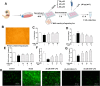
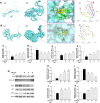
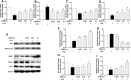
Dihydromyricetin (DHM) is a flavonoid found in vine tea that exhibits various pharmacological characteristics, including antibacterial, antiapoptotic, and antioxidant effects. Our previous study revealed that DHM can alleviate chicken hepatic injury, but the underlying mechanism has not been elucidated. To further investigate the protective mechanism of DHM, this study firstly predicted by network pharmacology that the potential regulatory pathways of DHM on hepatic injury. Subsequently, the experimental models were replicated in vivo and in vitro using Hy‒Line white broiler chickens and chicken primary hepatocytes treated with DHM and with/ without LPS. Network pharmacology results showed that the effect of DHM on hepatic injury might be related to oxidative stress and mitochondrial function. Further experiments showed that DHM significantly reduced LPS‒elicited serum ALT and AST activities, promoted antioxidant enzyme activities and scavenged ROS in chicken liver or primary hepatocytes. Molecular docking studies showed that DHM could directly bind to Nrf2 and Keap1. Furthermore, DHM treatment regulated the expression of Nrf2 and Keap1, thereby upregulating the downstream expression of antioxidant factors, including HO‒1 and NQO1, in vivo and in vitro. Moreover, DHM modulated the expression of mitochondrial dynamics related factors, including Mfn1/2, Opa1, Drp1, and Fis1, meanwhile, DHM ameliorated mitochondrial structural damage and increased the MMP. Overall, these results suggested that DHM activated the Nrf2/Keap1 pathway and regulated the balance between mitochondrial fusion and fission, ultimately alleviating chicken hepatic injury induced by LPS.
Keywords: Dihydromyricetin; Nrf2/Keap1 pathway, Mitochondrial dynamics, Oxidative stress, Hepatic injury.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Dihydromyricetin attenuates intervertebral disc degeneration by inhibiting NLRP3 inflammasome activation via the Keap1/Nrf2/HO-1 pathway.Eur J Pharmacol. 2025 Jul 5;998:177501. doi: 10.1016/j.ejphar.2025.177501. Epub 2025 Mar 8. Eur J Pharmacol. 2025. PMID: 40058758
-
Bamboo leaf flavonoids ameliorate cyclic heat stress-induced oxidative damage in broiler liver through activation of Keap1-Nrf2 signaling pathway.Poult Sci. 2025 Apr;104(4):104952. doi: 10.1016/j.psj.2025.104952. Epub 2025 Feb 26. Poult Sci. 2025. PMID: 40043675 Free PMC article.
-
Modulation of keap-1/Nrf2/HO-1 and NF-ĸb/caspase-3 signaling pathways by dihydromyricetin ameliorates sodium valproate-induced liver injury.Arch Biochem Biophys. 2024 Aug;758:110084. doi: 10.1016/j.abb.2024.110084. Epub 2024 Jul 4. Arch Biochem Biophys. 2024. PMID: 38971420
-
Unveiling the hepatoprotective mechanisms of Desmodium heterocarpon (L.) DC: Novel flavonoid identification and Keap1/Nrf2 pathway activation.Phytomedicine. 2025 Jan;136:156323. doi: 10.1016/j.phymed.2024.156323. Epub 2024 Dec 14. Phytomedicine. 2025. PMID: 39706064
-
Taraxasterol alleviates aflatoxin B1-induced liver damage in broiler chickens via regulation of oxidative stress, apoptosis and autophagy.Ecotoxicol Environ Saf. 2023 Feb;251:114546. doi: 10.1016/j.ecoenv.2023.114546. Epub 2023 Jan 14. Ecotoxicol Environ Saf. 2023. PMID: 36646010
References
-
- Ahmad A., Wali A.F., Rehman M.U., Khan A., Raish M., Kazi M., Alnemer O., Rao P.G.M. Therapeutic potential of rhododendron arboreum polysaccharides in an animal model of lipopolysaccharide‒inflicted oxidative stress and systemic inflammation. Molecules. 2020;25(24) doi: 10.3390/molecules25246045. - DOI - PMC - PubMed
-
- Chang Y., Yuan L., Liu J., Muhammad I., Cao C., Shi C., Zhang Y., Li R., Li C., Liu F. Dihydromyricetin attenuates Escherichia coli lipopolysaccharide-induced ileum injury in chickens by inhibiting NLRP3 inflammasome and TLR4/NF-κb signalling pathway. Vet. Res. 2020;51:72. doi: 10.1186/s13567-020-00796-8. - DOI - PMC - PubMed
-
- Chen F., Li B., Li W., Chen W., Huang Y., Tian Y., Yang B., Yuan M., Xu D., Cao N. Polysaccharide of atractylodes macrocephala koidz alleviate lipopolysaccharide-stimulated liver inflammation injury of goslings through miR-223/NLRP3 axis. Poult. Sci. 2023;102(1) doi: 10.1016/j.psj.2022.102285. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

