A live attenuated SARS-CoV-2 vaccine constructed by dual inactivation of NSP16 and ORF3a
- PMID: 40132472
- PMCID: PMC11985078
- DOI: 10.1016/j.ebiom.2025.105662
A live attenuated SARS-CoV-2 vaccine constructed by dual inactivation of NSP16 and ORF3a
Abstract
Background: Live attenuated vaccines against SARS-CoV-2 activate all phases of host immunity resembling a natural infection and they block viral transmission more efficiently than existing vaccines in human use. In our prior work, we characterised an attenuated SARS-CoV-2 variant, designated d16, which harbours a D130A mutation in the NSP16 protein, inactivating its 2'-O-methyltransferase function. The d16 variant has demonstrated an ability to induce both mucosal and sterilising immunity in animal models. However, further investigation is required to identify any additional modifications to d16 that could mitigate concerns regarding potential virulence reversion and the suboptimal regulation of the proinflammatory response.
Methods: Mutations were introduced into molecular clone of SARS-CoV-2 and live attenuated virus was recovered from cultured cells. Virological, biochemical and immunological assays were performed in vitro and in two animal models to access the protective efficacies of the candidate vaccine strain.
Findings: Here we describe evaluation of a derivative of d16. We further modified the d16 variant by inverting the open reading frame of the ORF3a accessory protein, resulting in the d16i3a strain. This modification is anticipated to enhance safety and reduce pathogenicity. d16i3a appeared to be further attenuated in hamsters and transgenic mice compared to d16. Intranasal vaccination with d16i3a stimulated humoural, cell-mediated and mucosal immune responses, conferring sterilising protection against SARS-CoV-2 Delta and Omicron variants in animals. A version of d16i3a expressing the XBB.1.16 spike protein further expanded the vaccine's protection spectrum against circulating variants. Notably, this version has demonstrated efficacy as a booster in hamsters, providing protection against Omicron subvariants and achieving inhibition of viral transmission.
Interpretation: Our work established a platform for generating safe and effective live attenuated vaccines by dual inactivation of NSP16 and ORF3a of SARS-CoV-2.
Funding: This work was supported by National Key Research and Development Program of China (2021YFC0866100, 2023YFC3041600, and 2023YFE0203400), Hong Kong Health and Medical Research Fund (COVID190114, CID-HKU1-9, and 23220712), Hong Kong Research Grants Council (C7142-20GF and T11-709/21-N), Hong Kong Innovation and Technology Commission grant (MHP/128/22), Guangzhou Laboratory (EKPG22-01) and Health@InnoHK (CVVT). Funding sources had no role in the writing of the manuscript or the decision to submit it for publication.
Keywords: COVID-19; Live attenuated vaccine; NSP16; ORF3a; SARS-CoV-2; Sterilising immunity.
Copyright © 2025 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests M.Z. and Y.Z. are employees of Sinopharm.
Figures
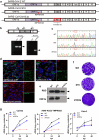
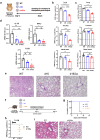
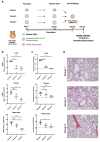
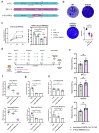
 ) highlights the D130A mutation in NSP16. (b) Genotyping to verify the inverted ORF3a sequence in d16i3a. P1, P2, and P3 indicate the positions of the three primers on SARS-CoV-2 genome. Black arrows indicate the expected bands. (c) Sequencing results of WT, d16 and d16i3a. The desired point mutation in NSP16 was highlighted by the red box. (d) Immunofluorescent staining. VeroE6-TMPRSS2 cells infected with WT, d16 or d16i3a were fixed at 24 h post-infection (hpi), and SARS-CoV-2 N (red) and ORF3a (green) proteins were detected. Cellular nuclei were stained with DAPI (blue). (e) Western blot analysis. Lysates of VeroE6-TMPRSS2 cells infected with WT, d16 or d16i3a at 0.1 MOI were collected at 24 hpi and probed with RBD- and ORF3a-specific antibodies. (f) Plaque phenotype. VeroE6 cells were infected with the indicated viruses. Cells were fixed and stained with 1% crystal violet. (g) CaCO2, A549-ACE2-TMPRSS2, and Calu-3 cells were infected with WT (black), d16 (blue) or d16i3a (pink) at 0.01 MOI. Viral RNA (vRNA) of the RNA-dependent RNA polymerase (RdRP) region in the supernatant was quantified by RT-qPCR.
) highlights the D130A mutation in NSP16. (b) Genotyping to verify the inverted ORF3a sequence in d16i3a. P1, P2, and P3 indicate the positions of the three primers on SARS-CoV-2 genome. Black arrows indicate the expected bands. (c) Sequencing results of WT, d16 and d16i3a. The desired point mutation in NSP16 was highlighted by the red box. (d) Immunofluorescent staining. VeroE6-TMPRSS2 cells infected with WT, d16 or d16i3a were fixed at 24 h post-infection (hpi), and SARS-CoV-2 N (red) and ORF3a (green) proteins were detected. Cellular nuclei were stained with DAPI (blue). (e) Western blot analysis. Lysates of VeroE6-TMPRSS2 cells infected with WT, d16 or d16i3a at 0.1 MOI were collected at 24 hpi and probed with RBD- and ORF3a-specific antibodies. (f) Plaque phenotype. VeroE6 cells were infected with the indicated viruses. Cells were fixed and stained with 1% crystal violet. (g) CaCO2, A549-ACE2-TMPRSS2, and Calu-3 cells were infected with WT (black), d16 (blue) or d16i3a (pink) at 0.01 MOI. Viral RNA (vRNA) of the RNA-dependent RNA polymerase (RdRP) region in the supernatant was quantified by RT-qPCR.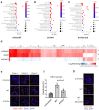


Similar articles
-
Nasal prevention of SARS-CoV-2 infection by intranasal influenza-based boost vaccination in mouse models.EBioMedicine. 2022 Jan;75:103762. doi: 10.1016/j.ebiom.2021.103762. Epub 2021 Dec 21. EBioMedicine. 2022. PMID: 34942445 Free PMC article.
-
Intranasal administration of a single dose of a candidate live attenuated vaccine derived from an NSP16-deficient SARS-CoV-2 strain confers sterilizing immunity in animals.Cell Mol Immunol. 2022 May;19(5):588-601. doi: 10.1038/s41423-022-00855-4. Epub 2022 Mar 29. Cell Mol Immunol. 2022. PMID: 35352010 Free PMC article.
-
A safe, effective and adaptable live-attenuated SARS-CoV-2 vaccine to reduce disease and transmission using one-to-stop genome modifications.Nat Microbiol. 2024 Aug;9(8):2099-2112. doi: 10.1038/s41564-024-01755-1. Epub 2024 Jul 12. Nat Microbiol. 2024. PMID: 38997518 Free PMC article.
-
SARS-CoV-2 Proteins: Are They Useful as Targets for COVID-19 Drugs and Vaccines?Curr Mol Med. 2022;22(1):50-66. doi: 10.2174/1566524021666210223143243. Curr Mol Med. 2022. PMID: 33622224 Review.
-
Severe acute respiratory syndrome-coronavirus-2 spike (S) protein based vaccine candidates: State of the art and future prospects.Rev Med Virol. 2021 May;31(3):e2183. doi: 10.1002/rmv.2183. Epub 2020 Oct 15. Rev Med Virol. 2021. PMID: 33594794 Free PMC article. Review.
References
-
- Scott L., Hsiao N.Y., Moyo S., et al. Track Omicron's spread with molecular data. Science. 2021;374:1454–1455. - PubMed
-
- Shuai H., Chan J.F.W., Hu B., et al. Attenuated replication and pathogenicity of SARS-CoV-2 B.1.1.529 Omicron. Nature. 2022;603:693–699. - PubMed
-
- Yuan S., Ye Z.W., Liang R., et al. Pathogenicity, transmissibility, and fitness of SARS-CoV-2 Omicron in Syrian hamsters. Science. 2022;377:428–433. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

