Rapid ER remodeling induced by a peptide-lipid complex in dying tumor cells
- PMID: 40132886
- PMCID: PMC11938384
- DOI: 10.26508/lsa.202403114
Rapid ER remodeling induced by a peptide-lipid complex in dying tumor cells
Abstract
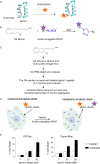
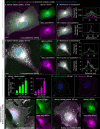
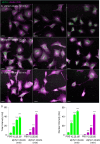
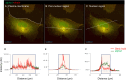
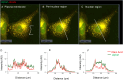
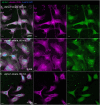
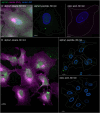
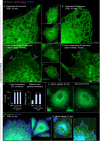
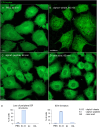
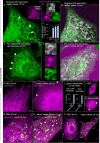
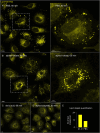
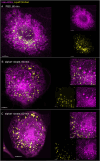
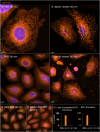
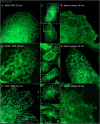
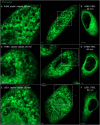
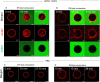
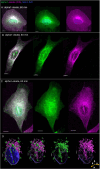
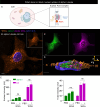
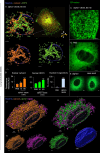
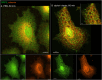
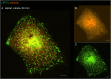
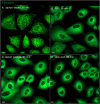
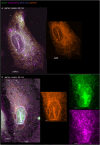
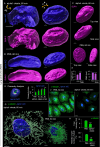
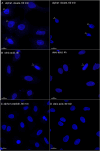
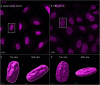
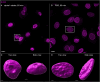
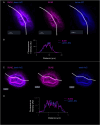
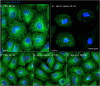
The membranous ER spans the entire cell, creating a network for the biosynthesis of proteins and lipids, cell-wide communication, and nuclear delivery of molecules, including therapeutic agents. Here, we identify a novel ER response triggered by the tumoricidal complex alpha1-oleate, defined by a loss of peripheral ER structure, extensive ER vesiculation. Alpha1-oleate was present in the ER-derived vesicle membranes, also decorated by ER-resident and ER-interacting proteins, calnexin and ORP3, and in their lumen, also enriched for KDEL, confirming their ER origin distinct from lipid droplets. Rapid nuclear uptake of the complex constituents resulted in diffuse nuclear staining, and the asymmetrical perinuclear enrichment of the collapsing ER with its content of alpha1-oleate created large invaginations lined by the ER, inner nuclear membrane markers, and lamin nucleoskeleton. In parallel, a change in nuclear shape resulted in a volcano-like structure. This newly discovered, potent ER response to alpha1-oleate may have evolved to package ER-associated cellular contents in the nuclei of dying tumor cells, thus sequestering toxic cell debris associated with apoptotic cell death.
© 2025 Sabari et al.
Conflict of interest statement
C Svanborg and I Ambite hold shares in HAMLET BioPharma, as a representative of scientists in the HAMLET group. Patents protecting the use of the alpha1-oleate complex have been granted. Other authors declare no conflict of interest.
Figures















References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
