Tissue-specific properties of type 1 dendritic cells in lung cancer: implications for immunotherapy
- PMID: 40132908
- PMCID: PMC11938230
- DOI: 10.1136/jitc-2024-010547
Tissue-specific properties of type 1 dendritic cells in lung cancer: implications for immunotherapy
Erratum in
-
Correction: Tissue-specific properties of type 1 dendritic cells in lung cancer: implications for immunotherapy.J Immunother Cancer. 2025 Apr 5;13(4):e010547corr1. doi: 10.1136/jitc-2024-010547corr1. J Immunother Cancer. 2025. PMID: 40187757 Free PMC article. No abstract available.
Abstract
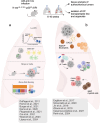
Checkpoint inhibitors have led to remarkable benefits in non-small cell lung cancer (NSCLC), yet response rates remain below expectations. High-dimensional analysis and mechanistic experiments in clinical samples and relevant NSCLC models uncovered the immune composition of lung cancer tissues, providing invaluable insights into the functional properties of tumor-infiltrating T cells and myeloid cells. Among myeloid cells, type 1 conventional dendritic cells (cDC1s) stand out for their unique ability to induce effector CD8 T cells against neoantigens and coordinate antitumoral immunity. Notably, lung resident cDC1 are particularly abundant and long-lived and express a unique tissue-specific gene program, underscoring their central role in lung immunity. Here, we discuss recent insights on the induction and regulation of antitumoral T cell responses in lung cancer, separating it from the tissue-agnostic knowledge generated from heterogeneous tumor models. We focus on the most recent studies dissecting functional states and spatial distribution of lung cDC1 across tumor stages and their impact on T cell responses to neoantigens. Finally, we highlight relevant gaps and emerging strategies to harness lung cDC1 immunostimulatory potential.
Keywords: Dendritic; Immunotherapy; Lung Cancer.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures



Similar articles
-
Vaccine efficacy against primary and metastatic cancer with in vitro-generated CD103+ conventional dendritic cells.J Immunother Cancer. 2020 Apr;8(1):e000474. doi: 10.1136/jitc-2019-000474. J Immunother Cancer. 2020. PMID: 32273347 Free PMC article.
-
Are Conventional Type 1 Dendritic Cells Critical for Protective Antitumor Immunity and How?Front Immunol. 2019 Feb 12;10:9. doi: 10.3389/fimmu.2019.00009. eCollection 2019. Front Immunol. 2019. PMID: 30809220 Free PMC article. Review.
-
TCF1-positive and TCF1-negative TRM CD8 T cell subsets and cDC1s orchestrate melanoma protection and immunotherapy response.J Immunother Cancer. 2024 Jul 5;12(7):e008739. doi: 10.1136/jitc-2023-008739. J Immunother Cancer. 2024. PMID: 38969523 Free PMC article.
-
Effective cancer immunotherapy by natural mouse conventional type-1 dendritic cells bearing dead tumor antigen.J Immunother Cancer. 2019 Apr 8;7(1):100. doi: 10.1186/s40425-019-0565-5. J Immunother Cancer. 2019. PMID: 30961656 Free PMC article.
-
Dendritic cell-based immunotherapy in non-small cell lung cancer: a comprehensive critical review.Front Immunol. 2024 Sep 6;15:1376704. doi: 10.3389/fimmu.2024.1376704. eCollection 2024. Front Immunol. 2024. PMID: 39308861 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
