mTOR inhibition modulates vaccine-induced immune responses to generate memory T cells in patients with solid tumors
- PMID: 40132910
- PMCID: PMC11956311
- DOI: 10.1136/jitc-2024-010408
mTOR inhibition modulates vaccine-induced immune responses to generate memory T cells in patients with solid tumors
Abstract
Background: Perturbation of the mechanistic target of rapamycin (mTOR) pathway can instruct effector versus memory cell fate of tumor antigen-specific T cells in preclinical models. In this study, we sought to understand the impact of rapamycin (sirolimus), an mTOR inhibitor, on reprogramming vaccine-induced T cells to enhance memory responses in patients with solid tumors following completion of their standard therapy.
Methods: We conducted three phase I clinical trials employing New York esophageal squamous cell carcinoma-1 (NY-ESO-1) vaccination approaches, with or without schedule-varied rapamycin. T cell phenotypes, functions, and Vβ usage in peripheral blood were analyzed to ask whether rapamycin influenced the generation of vaccine-induced T cells with memory attributes.
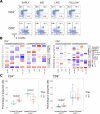
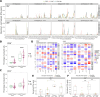
Results: The addition of rapamycin to all vaccination approaches was safe and well tolerated. Immediate (days 1-14 postvaccination) or delayed (days 15-28 postvaccination) administration of rapamycin led to a significant increase in the generation of vaccine-induced NY-ESO-1-specific T cells exhibiting central memory phenotypes (CD45RO+CD45RA- CCR7+). Moreover, delayed administration resulted in a greater than threefold (p=0.025) and eightfold (p=0.005) increase in the frequency of NY-ESO-1-specific CD4+ T and CD8+ T cells respectively at the time of long-term follow-up, compared with its immediate usage.
Conclusion: Our novel finding is that delayed administration of rapamycin to patients during the contraction phase of vaccine-induced antitumor immune responses was particularly effective in increasing the frequency of memory T cells up to 1 year postvaccination in patients with solid tumors. Further studies are warranted to identify the impact of this approach on the durability of clinical remission.
Trial registration number: NCT00803569, NCT01536054, NCT01522820.
Keywords: Immunotherapy; Memory; Ovarian Cancer; T cell; Vaccine.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: KO and RK are co-founders of Tactiva Therapeutics. The other authors have declared no conflict of interest exists.
Figures




References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
