Decreased practice effects in cognitively unimpaired amyloid betapositive individuals: a multicenter, longitudinal, cohort study
- PMID: 40133248
- PMCID: PMC11936764
- DOI: 10.1002/alz.70016
Decreased practice effects in cognitively unimpaired amyloid betapositive individuals: a multicenter, longitudinal, cohort study
Abstract
Introduction: We aimed to determine whether cognitively unimpaired (CU) amyloid- beta-positive (Aβ+) individuals display decreased practice effects on serial neuropsychological testing.
Methods: We included 209 CU participants from three research centers, 157 Aβ- controls and 52 Aβ+ individuals. Participants underwent neuropsychological assessment at baseline and annually during a 2-year follow-up. We used linear mixed-effects models to analyze cognitive change over time between the two groups, including time from baseline, amyloid status, their interaction, age, sex, and years of education as fixed effects and the intercept and time as random effects.
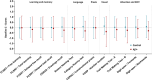
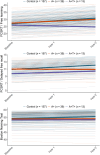
Results: The Aβ+ group showed reduced practice effects in verbal learning (β = -1.14, SE = 0.40, p = 0.0046) and memory function (β = -0.56, SE = 0.19, p = 0.0035), as well as in language tasks (β = -0.59, SE = 0.19, p = 0.0027).
Discussion: Individuals with normal cognition who are in the Alzheimer's continuum show decreased practice effects over annual neuropsychological testing. Our findings could have implications for the design and interpretation of primary prevention trials.
Highlights: This was a multicenter study on practice effects in asymptomatic Aβ+ individuals. We used LME models to analyze cognitive trajectories across multiple domains. Practice-effects reductions might be an indicator of subtle cognitive decline. Implications on clinical and research settings within the AD field are discussed.
Keywords: Alzheimer's disease; cognition; early detection; neuropsychological assessment; practice effects; subtle cognitive decline.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflicts of interest in connection with this paper. Author disclosures are available in the Supporting Information.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- JR22/00014/Instituto de Salud Carlos III (ISCIII)
- JR20/0018/Instituto de Salud Carlos III (ISCIII)
- PI21/00791/Instituto de Salud Carlos III (ISCIII)
- PI24/00598/Instituto de Salud Carlos III (ISCIII)
- PI19/00745/Instituto de Salud Carlos III (ISCIII)
- GBHI ALZ UK-21-720973/Global Brain Health Institute, Alzheimer's Association, Alzheimer's Society
- AACSF-21-850193/Global Brain Health Institute, Alzheimer's Association, Alzheimer's Society
- AACSF-21-850193/Alzheimer's Association Clinician Scientist Fellowship Program
- Fundación CITA-Alzhéimer Fundazioa
- PI12/02262/Ministry of Health of Spain
- PI1500919/Ministry of Health of Spain
- S-PR12CH001/Basque Country Government
- S-PR13ZH001/Basque Country Government
LinkOut - more resources
Full Text Sources
Medical

