Performance of plasma biomarkers for diagnosis and prediction of dementia in a Brazilian cohort
- PMID: 40133253
- PMCID: PMC11937383
- DOI: 10.1038/s41467-025-56756-3
Performance of plasma biomarkers for diagnosis and prediction of dementia in a Brazilian cohort
Abstract
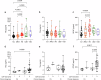
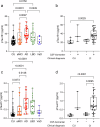
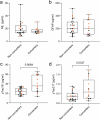
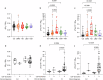
Despite remarkable progress in the biomarker field in recent years, local validation of plasma biomarkers of Alzheimer's disease (AD) and dementia is still lacking in Latin America. In this longitudinal cohort study of 145 elderly Brazilians, we assess the diagnostic performance of plasma biomarkers, based on clinical diagnosis and CSF biomarker positivity. Follow-up data of up to 4.7 years were used to determine performance in predicting diagnostic conversions. Participants were clinically categorized as cognitively unimpaired (n = 49), amnestic mild cognitive impairment (n = 29), AD (n = 38), Lewy body dementia (n = 22), or vascular dementia (n = 7). Plasma Tau, Aβ40, Aβ42, NfL, GFAP, pTau231, pTau181 and pTau217 were measured on the SIMOA HD-X platform. Plasma pTau217 showed excellent performance determining CSF biomarker status in the cohort, either alone (ROC AUC = 0.94, 95% CI: [0.88-1.00]) or as a ratio to Aβ42 (ROC AUC = 0.98, 95% CI: [0.94-1.00]). This study comprises an initial step towards local validation and adoption of dementia biomarkers in Brazil.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no conflicts of interest.
Figures

References
-
- Prince, M. et al. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer’s. Dement9, 63 (2013). - PubMed
-
- IBGE. Censo Demográfico 2022. https://agenciadenoticias.ibge.gov.br/agencia-noticias/2012-agencia-de-n... (2023).
-
- van Dyck, C. H. et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388, 9–21 (2023). - PubMed
-
- Karikari, T. K. et al. Blood phospho-tau in Alzheimer disease: analysis, interpretation, and clinical utility. Nat. Rev. Neurol.18, 400–418 (2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

