Identification of a SNAI1 enhancer RNA that drives cancer cell plasticity
- PMID: 40133308
- PMCID: PMC11937597
- DOI: 10.1038/s41467-025-58032-w
Identification of a SNAI1 enhancer RNA that drives cancer cell plasticity
Abstract
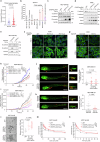
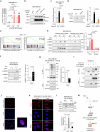
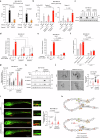
Enhancer RNAs (eRNAs) are a pivotal class of enhancer-derived non-coding RNAs that drive gene expression. Here we identify the SNAI1 enhancer RNA (SNAI1e; SCREEM2) as a key activator of SNAI1 expression and a potent enforcer of transforming growth factor-β (TGF-β)/SMAD signaling in cancer cells. SNAI1e depletion impairs TGF-β-induced epithelial-mesenchymal transition (EMT), migration, in vivo extravasation, stemness, and chemotherapy resistance in breast cancer cells. SNAI1e functions as an eRNA to cis-regulate SNAI1 enhancer activity by binding to and strengthening the enrichment of the transcriptional co-activator bromodomain containing protein 4 (BRD4) at the local enhancer. SNAI1e selectively promotes the expression of SNAI1, which encodes the EMT transcription factor SNAI1. Furthermore, we reveal that SNAI1 interacts with and anchors the inhibitory SMAD7 in the nucleus, and thereby prevents TGF-β type I receptor (TβRI) polyubiquitination and proteasomal degradation. Our findings establish SNAI1e as a critical driver of SNAI1 expression and TGF-β-induced cell plasticity.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Nieto, M. A., Huang, R. Y., Jackson, R. A. & Thiery, J. P. EMT: 2016. Cell166, 21–45 (2016). - PubMed
-
- Pastushenko, I. & Blanpain, C. EMT transition states during tumor progression and metastasis. Trends Cell Biol.29, 212–226 (2019). - PubMed
-
- Brabletz, T., Kalluri, R., Nieto, M. A. & Weinberg, R. A. EMT in cancer. Nat. Rev. Cancer18, 128–134 (2018). - PubMed
-
- Fan, C., Zhang, J., Hua, W. & ten Dijke, P. Biphasic role of TGF-β in cancer progression: from tumor suppressor to tumor promotor. In Reference Module in Biomedical Sciences (Elsevier, 2018).
-
- ten Dijke, P., Miyazono, K., Heldin, C. H. & Moustakas, A. Special issue: TGF-β and epithelial-mesenchymal transition in cancer. Semin. Cancer Biol.102-103, 1–3 (2024). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

