Flexible graphene-based neurotechnology for high-precision deep brain mapping and neuromodulation in Parkinsonian rats
- PMID: 40133322
- PMCID: PMC11937542
- DOI: 10.1038/s41467-025-58156-z
Flexible graphene-based neurotechnology for high-precision deep brain mapping and neuromodulation in Parkinsonian rats
Abstract
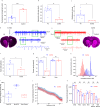
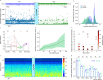
Deep brain stimulation (DBS) is a neuroelectronic therapy for the treatment of a broad range of neurological disorders, including Parkinson's disease. Current DBS technologies face important limitations, such as large electrode size, invasiveness, and lack of adaptive therapy based on biomarker monitoring. In this study, we investigate the potential benefits of using nanoporous reduced graphene oxide (rGO) technology in DBS, by implanting a flexible high-density array of rGO microelectrodes (25 µm diameter) in the subthalamic nucleus (STN) of healthy and hemi-parkinsonian rats. We demonstrate that these microelectrodes record action potentials with a high signal-to-noise ratio, allowing the precise localization of the STN and the tracking of multiunit-based Parkinsonian biomarkers. The bidirectional capability to deliver high-density focal stimulation and to record high-fidelity signals unlocks the visualization of local neuromodulation of the multiunit biomarker. These findings demonstrate the potential of bidirectional high-resolution neural interfaces to investigate closed-loop DBS in preclinical models.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: A.G., K.K. and J.A.G. declare that they hold interest in INBRAIN Neuroelectronics which has licensed the electrode technology used in this work. All other authors declare no competing interests.
Figures


References
-
- Benabid, A. L. Deep brain stimulation for Parkinson’s disease. Curr. Opin. Neurobiol.13, 696–706 (2003). - PubMed
-
- Lyons, K. E. & Pahwa, R. Deep Brain Stimulation and Essential Tremor. J. Clin. Neurophysiol.21, 2–5 (2004). - PubMed
-
- Krauss, J. K., Yianni, J., Loher, T. J. & Aziz, T. Z. Deep Brain Stimulation for Dystonia. J. Clin. Neurophysiol.21, 18–30 (2004). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

