Jamaican fruit bats' competence for Ebola but not Marburg virus is driven by intrinsic differences
- PMID: 40133326
- PMCID: PMC11937316
- DOI: 10.1038/s41467-025-58305-4
Jamaican fruit bats' competence for Ebola but not Marburg virus is driven by intrinsic differences
Abstract
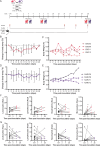
Ebola virus (EBOV) and Marburg virus (MARV) are zoonotic filoviruses that cause hemorrhagic fever in humans. Correlative data implicate bats as natural EBOV hosts, but neither a full-length genome nor an EBOV isolate has been found in any bats sampled. Here, we model filovirus infection in the Jamaican fruit bat (JFB), Artibeus jamaicensis, by inoculation with either EBOV or MARV through a combination of oral, intranasal, and subcutaneous routes. Infection with EBOV results in systemic virus replication and oral shedding of infectious virus. MARV replication is transient and does not shed. In vitro, JFB cells replicate EBOV more efficiently than MARV, and MARV infection induces innate antiviral responses that EBOV efficiently suppresses. Experiments using VSV pseudoparticles or replicating VSV expressing the EBOV or MARV glycoprotein demonstrate an advantage for EBOV entry and replication early, respectively, in JFB cells. Overall, this study describes filovirus species-specific phenotypes for both JFB and their cells.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Guito, J. C. et al. Asymptomatic infection of marburg virus reservoir bats is explained by a strategy of immunoprotective disease tolerance. Curr. Biol.31, 257–270.e255 (2021). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

