ChemoID-guided therapy improves objective response rate in recurrent platinum-resistant ovarian cancer randomized clinical trial
- PMID: 40133484
- PMCID: PMC11937309
- DOI: 10.1038/s41698-025-00874-0
ChemoID-guided therapy improves objective response rate in recurrent platinum-resistant ovarian cancer randomized clinical trial
Abstract
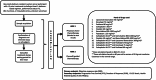
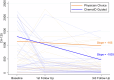
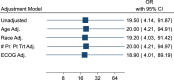
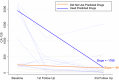
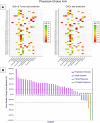
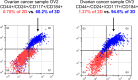
Patients with recurrent platinum-resistant ovarian cancer (PROC) have poor clinical outcomes, owing mainly to the presence of therapy-resistant cancer stem cells (CSCs). The NCT03949283 randomized clinical trial enrolled patients with recurrent PROC to receive ChemoID-guided chemotherapy or the best physician-choice regimen selected from the same list of thirteen mono or combination chemotherapies. The primary outcome was objective response rate (ORR) assessed on CT scans using the RECIST 1.1 criteria at 6 months follow-up. Subjects treated with the ChemoID assay had an ORR of 55% (CI95 39% - 73%), compared to 5% (CI95 0% - 11%) for those treated with physician's choice chemotherapy (p <0.0001). Secondary endpoints of duration of response (DOR) and progression-free survival (PFS) of subjects treated with chemotherapies guided by the ChemoID assay versus physician's choice chemotherapy were a median of 8 months vs. 5.5 months (p <0.0001), and 11.0 months (CI95 8.0- NA) vs 3.0 months (CI95 2.0- 3.5) with 27% of hazard ratio (CI95, 0.15-0.49; p <0.001), respectively.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: Drs. Claudio and Valluri reported ownership of intellectual property rights on the cancer stem cell platform technology licensed to Cordgenics, LLC. No other disclosures were reported for other authors.
Figures







References
-
- Hanker, L. C. et al. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann. Oncol.23, 2605–2612 (2012). - PubMed
-
- Djordjevic, B. et al. Current approach to epithelial ovarian cancer based on the concept of cancer stem cells. J. BUON17, 627–636 (2012). - PubMed
-
- Wang, X. et al. Eliminating ovarian cancer stem cells: a potential therapeutic target for ovarian cancer chemoresistance. Curr. Protein Pept. Sci.16, 270–278 (2015). - PubMed
-
- Markman, M. & Bookman, M. A. Second-line treatment of ovarian cancer. Oncologist5, 26–35 (2000). - PubMed
-
- Stuart, G. C. et al. 2010 Gynecologic Cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: report from the Fourth Ovarian Cancer Consensus Conference. Int J. Gynecol. Cancer21, 750–755 (2011). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

