Immunotherapy with low-dose IL-2 attenuates vascular injury in mice with diabetic and neovascular retinopathy by restoring the balance between Foxp3+ Tregs and CD8+ T cells
- PMID: 40133487
- PMCID: PMC12176913
- DOI: 10.1007/s00125-025-06412-8
Immunotherapy with low-dose IL-2 attenuates vascular injury in mice with diabetic and neovascular retinopathy by restoring the balance between Foxp3+ Tregs and CD8+ T cells
Abstract
Aims/hypothesis: Diabetic retinopathy features damage to the retinal microvasculature that causes vessels to leak and proliferate and can lead to vision loss and blindness. Inflammation contributes to the development of diabetic retinopathy, but little is known about the role of the adaptive immune system, including the benefits of augmenting the Forkhead box protein P3 (Foxp3) regulatory T cell (Treg) compartment. We aimed to determine whether treatment with low-dose IL-2 expands and activates Tregs and reduces CD8+ T cells in the retina, and attenuates retinal inflammation and vasculopathy in murine models of diabetic retinopathy and neovascular retinopathy.
Methods: Mouse models of streptozocin-induced diabetes and oxygen-induced retinopathy (OIR) were administered low-dose IL-2 (25,000 U) or vehicle (sterile water) by i.p. injection. Reporter mice expressing Foxp3 as a red fluorescent protein (RFP) conjugate or CD8 as a green fluorescent protein (GFP) conjugate were used to evaluate Foxp3+ Tregs and CD8+ T cells, respectively, in blood, lymphoid organs and retina using flow cytometry or confocal microscopy. Vasculopathy and the expression of angiogenic and inflammatory factors were assessed in the retina.
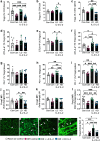
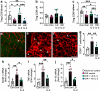
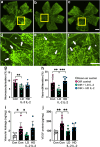
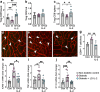
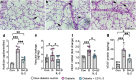
Results: Low-dose IL-2 significantly expanded CD4+CD25+Foxp3+ Tregs in the blood and spleen of mouse models of OIR and diabetes (1.4- to 1.9-fold increase, p<0.01). This expansion enhanced Treg functionality, increasing the expression of cytotoxic T-lymphocyte-associated protein4 (CTLA4), programmed cell death protein1 (PD1) and T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif (ITIM) domain (TIGIT), and increased the ratio of Tregs to CD8+ T cells. This was accompanied in the retina by a twofold increase in Foxp3+ Tregs (diabetes: 3.01 ± 0.41 vs 5.90 ± 1.25 cells per field, p<0.001; OIR: 4.41 ± 1.48 vs 10.05 ± 2.91 cells per field, p<0.001) and a reduction in CD8+ T cells (diabetes: 4.65 ± 0.58 vs 3.00 ± 0.81 cells per field, p<0.01; OIR: 5.51 ± 1.33 vs 3.17 ± 1.14 cells per field, p<0.01). Low-dose IL-2 reduced the levels of the potent inflammatory factors intercellular adhesion protein1 and TNF and the chemokine IFNγ-inducible protein10 (IP-10) in the retina. Importantly, low-dose IL-2 treatment effectively attenuated retinal vasculopathy, with marked reductions in acellular capillaries (diabetes: 0.48-fold decrease, p<0.001), neovascularisation (OIR: 0.68-fold decrease, p<0.01) and vascular leakage, and expression of vascular endothelial growth factor.
Conclusions/interpretation: This study highlights the therapeutic potential of low-dose IL-2 to reduce retinal inflammation and severe vascular injury by boosting Tregs and reducing CD8+ T cells and inflammatory factors.
Keywords: Angiogenesis; CD8+ T cells; Diabetic retinopathy; IL-2; Oxygen-induced retinopathy; Regulatory T cells; Tregs.
© 2025. The Author(s).
Conflict of interest statement
Data availability: The data from this study are available from the corresponding author on reasonable request. Funding: Open Access funding enabled and organized by CAUL and its Member Institutions. This research was supported by Breakthrough T1D (formerly known as JDRF) (ID #SRA-2020-968-M-B) and the National Health and Medical Research Council of Australia Ideas Grant scheme (1181462). DD was supported by a Breakthrough T1D Postdoctoral Fellowship (formerly known as JDRF; 1-PDF-2020-969-A-N). The funders were not involved in the design of the study, the collection, analysis and interpretation of data, or writing the report, and did not impose any restrictions regarding the publication of the report. Authors relationships and activities: All authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work. Contribution statement: DD and JLW-B conceived the study, drafted the manuscript, made substantial contributions to the acquisition, analysis and interpretation of the data, and revised and gave final approval to the manuscript. VS, AJo and AJa made substantial contributions to the acquisition, analysis and interpretation of data, and revised and gave final approval to the manuscript. DD and JLW-B are the guarantors of this work and take responsibility for the integrity of this work as a whole.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

