Regulating islet stress responses through CD47 activation
- PMID: 40133488
- PMCID: PMC12069481
- DOI: 10.1007/s00125-025-06409-3
Regulating islet stress responses through CD47 activation
Abstract
Aims/hypothesis: Diabetes is a global health burden characterised by incremental beta cell loss. Islet transplantation is a recognised treatment for individuals with type 1 diabetes and hypoglycaemia unawareness but broader application is constrained by limited islet survival and function post-transplantation. The underlying molecular mechanisms that induce beta cell dysfunction and demise remain unclear, and therapeutic agents that protect against cellular loss and maintain insulin secretion are in demand as potential treatment options. CD47 is a cell surface protein implicated in cellular stress responses but its role in beta cell function remains relatively unexplored. We hypothesised that modulating CD47 expression would demonstrate a cytoprotective effect in beta cells.
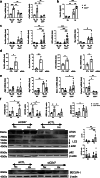
Methods: We used primary murine islets with/without genetic deletion of CD47, as well as human islets and MIN6 cells subjected to pharmacological disruption of CD47 signalling (siRNA or blocking antibody). Metabolic stress was induced in cells by exposure to hypoxia, hyperglycaemia or thapsigargin, and markers of the unfolded protein response, cell survival and insulin secretory function were assessed. Human pancreases from individuals with and without diabetes were examined for evidence of CD47 signalling.
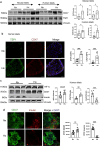
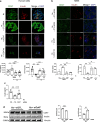
Results: Expression of CD47 and its high affinity ligand thrombospondin-1 (TSP1) was robustly upregulated by exogenous stressors. Limiting CD47 signalling improved markers of senescence, apoptosis, endoplasmic reticulum stress, unfolded protein response, self-renewal and autophagy, and maintained insulin secretory responses. We also found concurrent upregulated expression of CD47 and senescence markers in the endocrine pancreas of aged donors and those with type 2 diabetes. Both CD47 and TSP1 expression were increased in pancreases of humans with type 1 diabetes, as were plasma levels of TSP1.
Conclusions/interpretation: Our study provides key insights into the essential role of CD47 as a novel regulator of islet dysfunction, regulating cytoprotective responses to stress. CD47 may contribute to beta cell damage during the development of diabetes and failure of islet transplant function. Therefore, limiting CD47 activation may be a potential therapeutic tool in conditions where islet function is inadequate.
Keywords: CD47; Diabetes mellitus; ER stress; Hypoxia; Islet transplantation; Islets; Thrombospondin-1.
© 2025. The Author(s).
Conflict of interest statement
Data availability: This manuscript used data acquired from the Human Pancreas Analysis Program (HPAP-RRID:SCR_016202) database ( https://hpap.pmacs.upenn.edu/ ), a Human Islet Research Network (RRID:SCR_014393) consortium (UC4-DK-112217, U01-DK-123594, UC4-DK-112232 and U01-DK-123716). Funding: Open Access funding enabled and organized by CAUL and its Member Institutions. This work was supported by a JDRF Australia top-up scholarship to AK (SC4325), a National Health Medical Research Council grant (GNT2007991) to NMR, a Diabetes Australia General Grant to NMR (Y23G-ROGN) and to KG (Y21G-GHIK) and a Ramaciotti Foundation Health Investment Grant (2020HIG03) to NMR and KG. Authors’ relationships and activities: KG and NMR are named as inventors on a worldwide patent ‘Modulating insulin expression and production by targeting CD47’, publication no. WO/2022/087658. KG and NMR are not involved in any consulting services related to the current work. The authors declare that there are no other relationships or activities that might bias, or be perceived to bias, their work. Contribution statement: The study was conceptualised by NMR and KG. Experiments were performed by AK, MA, VC, SC, SMJ, PMM, KG and NMR. Bioinformatics analysis was performed by HR and EP. The methodology used was defined by AK, MA, KG and NMR. All authors were involved in data acquisition and analysis. The original draft of the manuscript was written by NMR, and all authors critically reviewed the manuscript and approved the final version. NMR is responsible for the integrity of the work as a whole.
Figures





References
-
- The European Nicotinamide Diabetes Intervention Trial (ENDIT) Group, Bingley PJ, Gale EAM et al (2006) Progression to type 1 diabetes in islet cell antibody-positive relatives in the European Nicotinamide Diabetes Intervention Trial: the role of additional immune, genetic and metabolic markers of risk. Diabetologia 49(5):881–890. 10.1007/s00125-006-0160-4 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

