[18F]FluorThanatrace PET imaging as a biomarker of response to PARP inhibitors in breast cancer
- PMID: 40133542
- PMCID: PMC11937411
- DOI: 10.1038/s43856-025-00791-0
[18F]FluorThanatrace PET imaging as a biomarker of response to PARP inhibitors in breast cancer
Abstract
Background: Poly (ADP-ribose) polymerase inhibitors (PARPi) are approved for Breast Cancer gene (BRCA)-mutant HER2- breast cancer, and there is clinical interest in expanding indications to include homologous recombination deficient (HRD) breast cancers. Yet, response in these populations remains variable, suggesting clinical utility in developing a better biomarker to select patients for PARPi and predict response. Here, we evaluate a radiolabeled PARPi, [18F]FluorThanatrace ([18F]FTT), as a functional biomarker of PARPi response in breast cancer.
Methods: A single-arm prospective observational trial was conducted at the University of Pennsylvania. [18F]FTT-PET uptake was measured in 24 women with untreated primary breast cancer and correlated with tumor HRD score. In a separate cohort of ten subjects with metastatic HER- breast cancer, [18F]FTT-PET uptake was measured at baseline and after a short interval on a PARPi (a measure of drug-target engagement) and correlated to progression free survival (PFS).
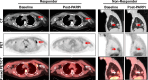
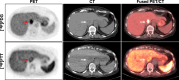
Results: Here we show that baseline [18F]FTT-PET uptake does not correlate to HRD tissue score, supporting that [18F]FTT provides distinct information from genetic features. Baseline [18F]FTT-PET uptake and the change in uptake from baseline to after PARPi initiation significantly correlates to PFS in woman with breast cancer who received a PARPi (ρ = 0.74, P = 0.023 and ρ = -0.86, P = 0.012, respectively).
Conclusions: These early results suggest the potential of [18F]FTT-PET to select patients for PARPi treatment and monitor in vivo pharmacodynamics after therapy start. Absence of association with HRD scores supports [18F]FTT uptake as a novel measure that may be leveraged as a biomarker. Further studies are warranted.
Plain language summary
PARP inhibitors are an effective treatment for breast cancer; however, do not work in all patients. Our goal is to identify individuals with breast cancer who are likely to respond to this treatment, so we do not administer the drug to those who will not benefit and could experience side effects. We gave people a probe that is visible by imaging and that binds to the PARP protein in the body. We found that the amount of probe taken up by a person’s tumor was an indicator of whether they would respond well to PARP inhibitor treatment. Using such a probe could help doctors make decisions about whether to treat breast cancer patients with PARP inhibitors.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: D.A.P. serves on the advisory board and licensed a patent to Trevarx Biomedical, a company with a license to [18F]FTT, the radiopharmaceutical investigated in this study. All other authors have declared that no conflicts of interest exist.
Figures



References
-
- Robson, M. et al. Olaparib for metastatic breast cancer in patients with a germline BRCA Mutation. N. Engl. J. Med.377, 523–533 (2017). - PubMed
-
- Eikesdal, H. P. et al. Olaparib monotherapy as primary treatment in unselected triple negative breast cancer. Ann. Oncol.32, 240–249 (2021). - PubMed
-
- Fasching, P. A. et al. Arbeitsgemeinschaft Gynakologische Onkologie B. Neoadjuvant paclitaxel/olaparib in comparison to paclitaxel/carboplatinum in patients with HER2-negative breast cancer and homologous recombination deficiency (GeparOLA study). Ann. Oncol.32, 49–57 (2021). - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

