Amikacin use in critically ill patients requiring renal replacement therapy: the AMIDIAL-ICU study
- PMID: 40133728
- PMCID: PMC11937451
- DOI: 10.1186/s13613-025-01461-z
Amikacin use in critically ill patients requiring renal replacement therapy: the AMIDIAL-ICU study
Abstract
Background: Acute kidney injury (AKI) requiring renal replacement therapy (RRT) is common in intensive care units (ICUs), yet optimal amikacin dosing in this context remains poorly understood.
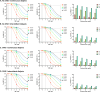
Methods: We conducted a prospective observational study across 18 French hospitals from April 2020 to January 2022. Adult ICU patients (aged > 18 years) receiving their first amikacin dose while on RRT were included. Data on demographics, RRT modalities, amikacin dosing, and therapeutic drug monitoring were collected. Using a pharmacokinetic modeling approach, we evaluated various amikacin regimens and simulated target attainment probabilities across different minimum inhibitory concentrations (MICs).
Results: A total of 111 patients were included, with approximately two-thirds receiving continuous RRT. The median amikacin dose was 27 (25-30) mg/kg. Amikacin peak (Cmax) and trough concentrations were monitored in 53 (47.8%) and 76 (68.5%) patients, respectively. Continuous RRT and a history of chronic kidney disease reduced dialytic clearance. For a MIC ≤ 4 mg/L, a 15 mg/kg amikacin dose achieved Cmax/MIC and AUC/MIC targets in ≥ 90% of patients on intermittent dialysis, while 20 mg/kg was required for those on continuous dialysis. For a MIC = 8 mg/L, a 30 mg/kg dose was necessary to achieve Cmax/MIC ≥ 8.
Conclusions: Our findings highlight suboptimal adherence to amikacin monitoring guidelines in ICU patients on RRT. Using pharmacokinetic modeling, we identified amikacin dosing recommendations ranging from 15 to 35 mg/kg to optimize efficacy and minimize risks, depending on MIC and dialysis modality.
Keywords: Amikacin; Aminoglycoside; Intensive care unit; Pharmacokinetic; Renal replacement therapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All subjects or their representatives provided informed consent to enroll. This study was approved by the ethics committee (CPP Sud Ouest et Outremer 2, 2020) and was registered on CinicalTrials.gov (NCT04322019). Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures
References
-
- Agence française de sécurité sanitaire des produits de santé. Update on good use of injectable aminoglycosides, Gentamycin, tobramycin, Netilmycin, Amikacin. Pharmacological properties, indications, dosage, and mode of administration, treatment monitoring. Med Mal Infect. 2012;42(7):301–8. - PubMed
-
- Marik PE. Aminoglycoside volume of distribution and illness severity in critically ill septic patients. Anaesth Intensive Care. 1993;21(2):172–3. - PubMed
-
- de Montmollin E, Bouadma L, Gault N, Mourvillier B, Mariotte E, Chemam S, et al. Predictors of insufficient Amikacin peak concentration in critically ill patients receiving a 25 mg/kg total body weight regimen. Intensive Care Med. 2014;40(7):998–1005. - PubMed
-
- Moore RD, Lietman PS, Smith CR. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;155(1):93–9. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

