Generalizability of tau and amyloid plasma biomarkers in Alzheimer's disease cohorts of diverse genetic ancestries
- PMID: 40133765
- PMCID: PMC11936763
- DOI: 10.1002/alz.14367
Generalizability of tau and amyloid plasma biomarkers in Alzheimer's disease cohorts of diverse genetic ancestries
Abstract
Introduction: Plasma phosphorylated threonine 181 of tau (pTau181) and amyloid beta (Aβ) are biomarkers for differential diagnosis and preclinical detection of Alzheimer disease (AD). Given differences in AD risk across diverse populations, the generalizability of existing biomarker data is not assured.
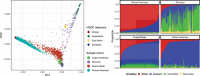
Methods: In 2086 individuals of diverse genetic ancestries (African American, Caribbean Hispanic, and Peruvian), we measured plasma pTau181 and Aβ42/Aβ40. Differences in biomarkers between cohorts and clinical diagnosis groups and the potential discriminative performance of the two biomarkers were assessed.
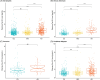
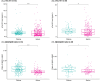
Results: pTau181 and Aβ42/Aβ40 were consistent across cohorts. Higher levels of pTau181 were associated with AD, while Aβ42/Aβ40 had minimal differences. Correspondingly, pTau181 had a greater predictive value than Aβ42/Aβ40; however, the area under the curve differed between cohorts.
Discussion: pTau181 as a plasma biomarker for clinical AD is generalizable across genetic ancestries, but its predictive value may vary. Combining genomic and biomarker data from diverse individuals will increase understanding of genetic risk and refine clinical diagnoses.
Highlights: This is a diverse ancestry study of plasma biomarkers for AD. Plasma biomarkers were assessed in African Americans, Caribbean Hispanics, and Peruvians. Biomarker levels were consistent across the diverse cohorts. Plasma phosphorylated tau was higher in AD in all cohorts. Plasma biomarker findings in diverse cohorts largely generalize with existing European studies.
Keywords: Alzheimer's disease; amyloid; diverse ancestry; plasma biomarkers; tau.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflicts of interest. Author disclosures are available in the Supporting Information.
Figures
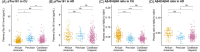


Update of
-
Generalizability of Tau and Amyloid Plasma Biomarkers in Alzheimer's Disease Cohorts of Diverse Genetic Ancestries.medRxiv [Preprint]. 2024 Apr 12:2024.04.10.24305617. doi: 10.1101/2024.04.10.24305617. medRxiv. 2024. Update in: Alzheimers Dement. 2025 Mar;21(3):e14367. doi: 10.1002/alz.14367. PMID: 38645114 Free PMC article. Updated. Preprint.
References
-
- 2023 Alzheimer's disease facts and figures. Alzheimers Dement. 2023;19:1598‐1695. - PubMed
MeSH terms
Substances
Grants and funding
- AG070864/National Institutes of Health, National Institute on Aging
- U19 AG074865/AG/NIA NIH HHS/United States
- RF1 AG070935/AG/NIA NIH HHS/United States
- AG070935/National Institutes of Health, National Institute on Aging
- AG072547/National Institutes of Health, National Institute on Aging
- AG084545/National Institutes of Health, National Institute on Aging
- R01 AG062268/AG/NIA NIH HHS/United States
- AG074865/National Institutes of Health, National Institute on Aging
- R01 AG070864/AG/NIA NIH HHS/United States
- R01 AG070935/AG/NIA NIH HHS/United States
- U01 AG079850/AG/NIA NIH HHS/United States
- U01 AG084545/AG/NIA NIH HHS/United States
- R56 AG072547/AG/NIA NIH HHS/United States
- R01 AG072547/AG/NIA NIH HHS/United States
- AG052410/National Institutes of Health, National Institute on Aging
- R01 MH120794/MH/NIMH NIH HHS/United States
- U01 AG052410/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

