Evaluating maternal serum sortilin levels: a potential biomarker for predicting preeclampsia
- PMID: 40133840
- PMCID: PMC11934441
- DOI: 10.1186/s12884-025-07452-z
Evaluating maternal serum sortilin levels: a potential biomarker for predicting preeclampsia
Abstract
Objective: To determine the role of sortilin in the pathogenesis of preeclampsia by examining serum sortilin levels in maternal blood.
Methods: This prospective case-control study was conducted from May to November 2023 at the Perinatology Clinic of Ankara Etlik City Hospital. The study cohort was divided into two groups: Group 1 consisted of 44 pregnant women diagnosed with preeclampsia, and Group 2 served as the control group, comprising 44 healthy pregnant women. The groups were matched individually, with controls selected based on similar maternal age and gestational age at the time of sample collection.
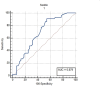
Results: Maternal sortilin levels were significantly elevated in preeclampsia patients compared to controls. Using a cut-off value of > 3.57 ng/mL, sortilin levels could distinguish preeclampsia cases with a sensitivity of 90.9%, a specificity of 45.5%, and an area under the curve (AUC) of 0.679 (p = 0.002). At a cut-off of > 3.57 ng/mL, it was significantly associated with composite adverse neonatal outcomes, with a sensitivity of 89.6%, a specificity of 36.1%, and an AUC of 0.620 (p = 0.045). In addition, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and protein in 24-hour urine, which are important components in the diagnosis and severity of preeclampsia, were significantly correlated maternal blood sortilin levels.
Conclusion: Our findings indicate that maternal sortilin levels are elevated in patients with preeclampsia compared to those in a healthy pregnant control group. Furthermore, maternal sortilin levels may predict adverse neonatal outcomes. In addition, sortilin levels are correlated key clinical markers of preeclampsia severity.
Keywords: Maternal blood; Neonatal outcomes; Perinatal outcomes; Preeclampsia; Sortilin.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study protocol was approved by the Ethics Committee of Ankara Etlik City Hospital (approval number: AESH-EK1-2023-253). The patients who participated in the study were informed and their written informed consent was obtained. This study complied with the provisions of the Declaration of Helsinki. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Dimitriadis E, Rolnik DL, Zhou W, Estrada-Gutierrez G, Koga K, Francisco RPV, et al. Pre-eclampsia. Nat Rev Dis Primer. 2023;9(1):8. - PubMed
-
- Rolnik DL, Nicolaides KH, Poon LC. Prevention of preeclampsia with aspirin. Am J Obstet Gynecol. 2022;226(2S):S1108–19. - PubMed
-
- Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381. - PubMed
-
- Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374(1):13–22. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

