Efficacy and acceptability of a self-collected medical grade tampon as a novel vaginal sample collection tool for the detection of HPV and STIs
- PMID: 40133975
- PMCID: PMC11938752
- DOI: 10.1186/s12905-025-03652-z
Efficacy and acceptability of a self-collected medical grade tampon as a novel vaginal sample collection tool for the detection of HPV and STIs
Abstract
Objective: Cervical cancer remains a significant health concern, particularly in low-income and middle-income countries (LMICs). This study aims to compare the efficacy and suitability of a self-collected tampon for the detection of human papillomavirus (HPV) and sexually transmitted infections (STIs) using qualitative TMA-based assays (Transcription Mediated Amplification; APTIMA® HPV, APTIMA® Combo 2 (CT/NG; AC2 from now on) and APTIMA®Bacterial Vaginosis (BV from now on). Additionally, we assess the acceptability of tampons as a self-collection tool.
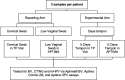
Methods: A cohort of 75 female participants aged 18-54 years was recruited through female-focused social networks. Participants provided informed consent and underwent both Health Care Workers (HCW-collected) and self-collected sample collection using the Daye Diagnostic Tampon. Samples were stored in ThinPrep Vials (TP Vial) or Aptima® Multitest Swab Collection Kit (APTIMA®) solutions. HPV and STI testing were performed using TMA-based assay on the fully automated Panther® Platform. Acceptability was assessed through a questionnaire with Likert-scale responses.
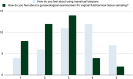
Results: The study involved 60 participants who completed the study (80% of recruited participants). The self-collected tampons showed sensitivity and specificity of 66.67% and 90.74% (when rinsed in TP Vial) and 83.33% and 85.42% (when rinsed in APTIMA®) for HPV detection, respectively. For bacterial vaginosis (BV) detection, the tampons exhibited sensitivity and specificity of 100.0% and 96.43% (TP Vial) and 88.89% and 98.04% (APTIMA), respectively. For detection of chlamydia and gonorrhoea (AC2), the sensitivity and specificity were 100.00% and 100.0% (TP Vial) and 100.00% and 98.31% (APTIMA), respectively. Participants expressed a preference for tampon self-collection over HCW-collected swabs (90%).
Conclusion: Self-collected tampons demonstrated promising diagnostic accuracy to HCW-collected swabs for HPV and STI detection. The tampon self-collection method was well-accepted and preferred by participants, suggesting its potential as an alternative screening tool, particularly in low-resource settings. Further research with larger and more diverse populations is recommended to validate these findings and inform tampon-based self-collection programs for cervical cancer screening. Randomised controlled trials and comparisons with gold standard methods would enhance validation.
Keywords: Acceptability; Cervical cancer; Diagnostic accuracy; HPV; STI; Self-collection; TMA; Tampon.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was conducted in compliance with ethical guidelines and principles established by the ICH Guideline for Good Clinical Practice & Declaration of Helsinki and Bulgarian Health Law. The research protocol was reviewed and approved by Ascendent Medical Center, Bulgaria before data collection commenced. All necessary measures were taken to ensure the protection of the rights, dignity, and well-being of the participants involved. Prior to participation, informed consent was obtained from all individuals included in this study. Participants were provided with a detailed explanation of the study objectives, procedures, potential risks and benefits, and their right to withdraw from the study at any point without consequences. Furthermore, they were assured of the confidentiality and anonymity of their personal information. To protect the privacy and confidentiality of the participants, all data collected during this study was coded and stored securely. Any identifying information was removed or anonymized to maintain the privacy of the participants. Only authorised researchers had access to the data, and the information obtained was solely used for the purposes of this study. This research adheres to the principles outlined in the ICH Guideline for Good Clinical Practice & Declaration of Helsinki. We recognise the importance of ethical considerations in research and strive to conduct studies that contribute to knowledge while respecting the rights and well-being of the participants involved. Consent for publication: Annes Day LTD consents to the publication of the study “Efficacy and Acceptability of Self-Collected Medical Grade Tampon as a Novel Tool for Vaginal Sample Collection Tool for the Detection of HPV and STIs.” Competing interests: The authors declare no competing interests.
Figures




References
-
- Aldunate M, Srbinovski D, Hearps AC, Latham CF, Ramsland PA, Gugasyan R, Cone RA, Tachedjian G. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front Physiol. 2015;6:164. - PMC - PubMed
-
- Aptima BV Clinical Trial Package Insert AW-23712–001, SanDiego, CA; Hologic Inc., 2020.
-
- Aptima Combo 2 package insert AW-19693–001, San Diego, CA; Hologic, Inc., 2020
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

