Innovative dual-gene delivery platform using miR-124 and PD-1 via umbilical cord mesenchymal stem cells and exosome for glioblastoma therapy
- PMID: 40134003
- PMCID: PMC11934454
- DOI: 10.1186/s13046-025-03336-4
Innovative dual-gene delivery platform using miR-124 and PD-1 via umbilical cord mesenchymal stem cells and exosome for glioblastoma therapy
Abstract
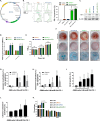
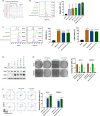
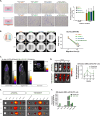
Addressing the challenges of identifying suitable targets and effective delivery strategies is critical in pursuing therapeutic solutions for glioblastoma (GBM). This study focuses on the therapeutic potential of microRNA-124 (miR-124), known for its tumor-suppressing properties, by investigating its ability to target key oncogenic pathways in GBM. The results reveal that CDK4 and CDK6-cyclin-dependent kinases that promote cell cycle progression-are significantly overexpressed in GBM brain samples, underscoring their role in tumor proliferation and identifying them as critical targets for miR-124 intervention. However, delivering miRNA-based therapies remains a major obstacle due to the instability of RNA molecules and the difficulty in achieving targeted, efficient delivery. To address these issues, this research introduces an innovative, non-viral dual-gene delivery platform that utilizes umbilical cord mesenchymal stem cells (UMSCs) and their exosomes to transport miR-124 and programmed cell death protein-1 (PD-1). The efficacy of this dual-gene delivery system was validated using an orthotopic GBM model, which closely mimics the tumor microenvironment seen in patients. Experimental results demonstrate that the UMSC/miR-124-PD-1 complex and its exosomes successfully induce apoptosis in GBM cells, significantly inhibiting tumor growth. Notably, these treatments show minimal cytotoxic effects on normal glial cells, highlighting their safety and selectivity. Moreover, the study highlights the immunomodulatory properties of UMSC/miR-124-PD-1 and its exosomes, enhancing the activation of immune cells such as T cells and dendritic cells, while reducing immunosuppressive cells populations like regulatory T cells and myeloid-derived suppressor cells. The orchestrated dual-gene delivery system by UMSCs and exosomes showcased targeted tumor inhibition and positive immune modulation, emphasizing its potential as a promising therapeutic approach for GBM.
Keywords: CDK4/6; Gene therapy; Glioblastoma; Umbilical cord mesenchymal stem cells; miR-124.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Studies involving animal subjects: The animal study conducted in this research was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at China Medical University, Taichung, Taiwan (ID: CMU CMUIACUC-2019-042). Studies involving human subjects: Opensource data was used. Opensource data was used. The collection of brain tumor samples was approved by the Office of Human Research at Taipei Medical University (ID: N201901041). Inclusion of identifiable human data: There is no inclusion of identifiable human data in the manuscript. Consent for publication: All authors have agreed to the publication of the manuscript. Competing interests: The authors declare no competing interests.
Figures



References
-
- Nozhat Z, Heydarzadeh S, Shahriari-Khalaji M, Wang S, Iqbal MZ, Kong X. Advanced biomaterials for human glioblastoma multiforme (GBM) drug delivery. Biomaterials Sci. 2023;11(12):4094–131. - PubMed
-
- Abdul-Al M, Saeinasab M, Zare A, Barati M, Shakeri S, Keykhosravi E, Momeni-Moghaddam M, Najafzadeh M, Keshel SH, Farzi G, Sefat F. Application of biomaterials for glioblastoma treatment: promises, advances, and challenges. Mater Today Commun. 2022;33:104562.
-
- Xu H-Z, Li T-F, Ma Y, Li K, Zhang Q, Xu Y-H, Zhang Y-C, Zhao L, Chen X. Targeted photodynamic therapy of glioblastoma mediated by platelets with photo-controlled release property. Biomaterials. 2022;290:121833. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

