In vivo synergistic enhancement of MIF-mediated inflammation in acute lung injury by the plant ortholog Arabidopsis MDL1
- PMID: 40134325
- PMCID: PMC11937861
- DOI: 10.1096/fj.202403301R
In vivo synergistic enhancement of MIF-mediated inflammation in acute lung injury by the plant ortholog Arabidopsis MDL1
Abstract
Recent research has uncovered Arabidopsis thaliana proteins that are similar to the human inflammatory cytokine MIF. Plant MIF/D-dopachrome tautomerase (D-DT)-like proteins (MDLs) can interact with human MIF, yet the significance of these findings in living organisms has not been investigated. Given MIF's key role in acute respiratory distress syndrome promoting pulmonary inflammation, pathology, and leukocyte infiltration, here we set out to investigate the interplay between MIF and MDL1, one of three A. thaliana MIF orthologs, in an in vivo mouse model of MIF-induced acute lung injury (ALI). Human MIF and MDL1 were administered to C57BL/6 mice via inhalation, individually or in combination. Inhalation of MIF promoted various parameters of lung injury as evaluated by flow cytometry, immunofluorescence microscopy, RT-qPCR, and ELISA, while MDL1 inhalation alone had no effect. Intriguingly, combined treatment with MIF and MDL1 synergistically enhanced pulmonary infiltration of neutrophils and monocytic cells, accompanied by an upregulation of pro-inflammatory cytokine genes. Thus, the plant-derived MIF ortholog MDL1 potentiates MIF-induced inflammation in ALI. These data support the growing evidence of interactions between plant-derived compounds and human inflammatory mediators and illustrate how they may impact human health.
Keywords: MDL; MIF; acute lung injury; atypical chemokine; plant MIF.
© 2025 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
J.B. is the inventor on patent applications related to anti‐MIF strategies. All other authors declare no competing interests.
Figures
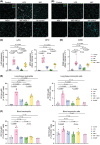
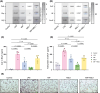
References
-
- Spiller L, Manjula R, Leissing F, et al. Plant MDL proteins synergize with the cytokine MIF at CXCR2 and CXCR4 receptors in human cells. Sci Signal. 2023;16:eadg2621. - PubMed
-
- Bernhagen J, Krohn R, Lue H, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13:587‐596. - PubMed
MeSH terms
Substances
Grants and funding
- BE 1977/10-1/Deutsche Forschungsgemeinschaft (DFG)
- BE1977/17-1/Deutsche Forschungsgemeinschaft (DFG)
- SFB1123-A3/Deutsche Forschungsgemeinschaft (DFG)
- SFB1123-A2/Deutsche Forschungsgemeinschaft (DFG)
- EXC2145SyNergy-ID390857198/Deutsche Forschungsgemeinschaft (DFG)
- PA861/15-1/Deutsche Forschungsgemeinschaft (DFG)
- PA861/24-1/Deutsche Forschungsgemeinschaft (DFG)
- STO1099/8-1/Deutsche Forschungsgemeinschaft (DFG)
- China Scholarship Council
- Metiphys scholarship of LMU Munich
- Knowledge Transfer Fund (KTF) of the LMU Munich-DFG excellence (LMUexc) program
- Friedrich-Baur-Foundation e.V.
- Open Philanthropy Project
- LMU Center for Advanced Sciences (CAS) program for visiting professors
LinkOut - more resources
Full Text Sources
Miscellaneous

