Tetraspanins affect membrane structures and the trafficking of molecular partners: what impact on extracellular vesicles?
- PMID: 40135387
- PMCID: PMC12203959
- DOI: 10.1042/BST20240523
Tetraspanins affect membrane structures and the trafficking of molecular partners: what impact on extracellular vesicles?
Abstract
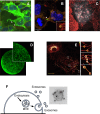
Tetraspanins are a family of 33 proteins in mammals believed to play a crucial role in the compartmentalization of various associated proteins within cells and membranes. Recent studies have elucidated the structure of several tetraspanin members, revealing that while the four transmembrane domains typically adopt a cone-shaped configuration in crystals, other conformations are also possible. This cone-shaped structure may explain why tetraspanins are often enriched in curved and tubular cellular structures, such as microvilli, tunneling nanotubes, retraction fibers, or at the site of virus budding, and may contribute to the formation or maintenance of these structures. Tetraspanins have also been detected on midbody remnants and migrasomes, as well as on extracellular vesicles (EVs), for which CD9, CD81, and CD63 are widely used as markers. Although their impact on certain membrane structures and their ability to regulate the function and trafficking of associated proteins would suggest a potential role of tetraspanins either in EV formation or in regulating their protein composition, or both, efforts to characterize these roles have been complicated by conflicting results. In line with the interaction of certain tetraspanins with cholesterol, two recent studies have suggested that the presence or organization of oxysterols and cholesterol in EVs may be regulated by Tspan6 and CD63, respectively, paving the way for further research on the influence of tetraspanins on the lipid composition of EVs.
Keywords: exosomes; extracellular vesicles; membrane dynamics; membrane proteins; microparticles; tetraspanins.
© 2025 The Author(s).
Conflict of interest statement
ER declares that there are no competing interests associated with the manuscript. PZ and CT are inventors on respectively 1 and 2 filed patents on the therapeutic use of EVs.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

