Color variation in radio-luminescence of P-dots doped with thermally activated delayed fluorescence molecules
- PMID: 40135457
- PMCID: PMC11938209
- DOI: 10.1039/d5cp00410a
Color variation in radio-luminescence of P-dots doped with thermally activated delayed fluorescence molecules
Abstract
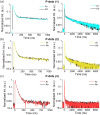
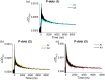
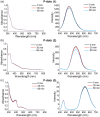
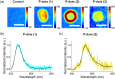
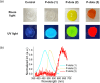
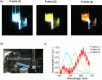
Thermally activated delayed fluorescence (TADF) materials possess exceptional photophysical properties. Organic scintillators utilizing TADF materials have shown great promise for applications requiring efficient radio-luminescence, owing to their high quantum efficiency and tunable emission properties. Previous studies demonstrated that polymer dots (P-dots) doped with TADF materials exhibit radio-luminescence under hard X-ray and electron beam excitation. However, the TADF materials used in these experiments were limited to limited color options, restricting their utility and hindering the exploration of multicolor radio-luminescence necessary for advanced applications. In this study, we successfully achieved multicolor radio-luminescence-blue, yellow, and red-by developing P-dots doped with TADF materials that emit across the visible spectrum. This breakthrough was demonstrated under excitation by hard X-rays, gamma rays, and electron beams. The ability to realize multicolor radio-luminescence is crucial, as it enables enhanced spatial and spectral resolution, which is vital for applications such as high-precision bio-imaging and multimodal sensing.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

