Induction of hepatitis B core protein aggregation targeting an unconventional binding site
- PMID: 40135596
- PMCID: PMC11942178
- DOI: 10.7554/eLife.98827
Induction of hepatitis B core protein aggregation targeting an unconventional binding site
Abstract
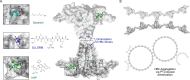
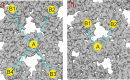
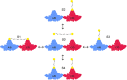
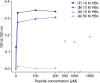
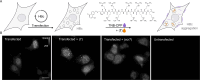
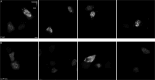
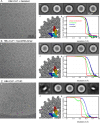
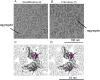
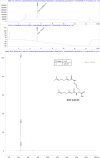
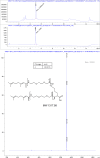
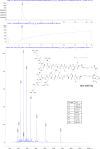
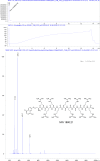
The hepatitis B virus (HBV) infection is a major global health problem, with chronic infection leading to liver complications and high death toll. Current treatments, such as nucleos(t)ide analogs and interferon-α, effectively suppress viral replication but rarely cure the infection. To address this, new antivirals targeting different components of the HBV molecular machinery are being developed. Here we investigated the hepatitis B core protein (HBc) that forms the viral capsids and plays a vital role in the HBV life cycle. We explored two distinct binding pockets on the HBV capsid: the central hydrophobic pocket of HBc-dimers and the pocket at the tips of capsid spikes. We synthesized a geranyl dimer that binds to the central pocket with micromolar affinity, and dimeric peptides that bind the spike-tip pocket with sub-micromolar affinity. Cryo-electron microscopy further confirmed the binding of peptide dimers to the capsid spike tips and their capsid-aggregating properties. Finally, we show that the peptide dimers induce HBc aggregation in vitro and in living cells. Our findings highlight two tractable sites within the HBV capsid and provide an alternative strategy to affect HBV capsids.
Keywords: CAMs; Cryo-EM; HBc; biochemistry; capsid assembly modulators; chemical biology; cryo-electron microscopy; hepatitis B core protein; hepatitis B virus; none; peptide.
Plain language summary
New and better strategies to treat hepatitis B are urgently needed. Many people worldwide remain unvaccinated against the disease, leaving them vulnerable to infection and serious liver problems. Children are particularly at risk of developing long-term illness. Treatments exist to help manage the condition, but they can rarely cure it. Hepatitis B virus is protected by a spiky shell called the capsid, made of HBc proteins. This structure is critical for survival, and therefore a promising therapeutic target. Current approaches rely on compounds disrupting the assembly or stability of this structure by binding onto the HBc protein. So far, most of these drug candidates target the same location at the base of the capsid’s spikes. Until now, other binding pockets on the capsid remained largely unexplored. To investigate whether these sites could be potential drug targets, Khayenko et al. developed two types of molecules, peptides and geranyl dimers, that could theoretically attach to the capsid – the former at the tip of the spikes and the latter in their middle section. In vitro and in living cells, the compounds not only latched onto these sites, but caused HBc proteins to clump together, preventing the capsid from forming properly. A similar mode of action is observed with existing drug candidates that, however, all bind to the pocket at the base of the spikes. These findings highlight alternative strategies for targeting hepatitis B. Future studies will need to determine how well these molecules work in clinical conditions, and whether they could complement or improve existing treatments.
© 2024, Khayenko, Makbul et al.
Conflict of interest statement
VK, CM, CS, NH, SK, BB, HM No competing interests declared
Figures








Update of
- doi: 10.1101/2024.04.29.591677
- doi: 10.7554/eLife.98827.1
- doi: 10.7554/eLife.98827.2
References
-
- Bonn B, Strängberg E, Uzelac I, Kirstgen M, Goldmann N, Glebe D, Geyer J, Lindström E. P15 The orally available sodium/taurocholate co-transporting polypeptide inhibitor A2342 blocks hepatitis B and D entry in vitro. Gut. 2022;71:A42. doi: 10.1136/gutjnl-2022-BASL.66. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

