Pathways underlying selective neuronal vulnerability in Alzheimer's disease: Contrasting the vulnerable locus coeruleus to the resilient substantia nigra
- PMID: 40135662
- PMCID: PMC11938114
- DOI: 10.1002/alz.70087
Pathways underlying selective neuronal vulnerability in Alzheimer's disease: Contrasting the vulnerable locus coeruleus to the resilient substantia nigra
Abstract
Introduction: Alzheimer's disease (AD) selectively affects certain brain regions, yet the mechanisms of selective vulnerability remain poorly understood. The neuromodulatory subcortical system, which includes nuclei exhibiting a range of vulnerability and resilience to AD-type degeneration, presents a framework for uncovering these mechanisms.
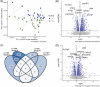
Methods: We leveraged transcriptomics and immunohistochemistry in paired samples from human post mortem tissue representing a vulnerable and resilient region-the locus coeruleus (LC) and substantia nigra (SN). These regions have comparable anatomical features but distinct vulnerability to AD.
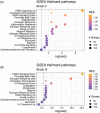
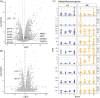
Results: We identified significant differences in cholesterol homeostasis, antioxidant pathways, KRAS signaling, and estrogen signaling at a bulk transcriptomic level. Notably, evidence of sigma-2 receptor upregulation was detected in the LC.
Discussion: Our findings highlight pathways differentiating the LC and SN, potentially explaining the LC's selective vulnerability in AD. Such pathways offer potential targets of disease-modifying therapies for AD.
Highlights: Intraindividual comparative RNAseq was used to study selective vulnerability. Metallothionein genes are significantly enriched in the substantia nigra. Cholesterol homeostatic genes are significantly enriched in the locus coeruleus. The locus coeruleus is likely more susceptible to toxic amyloid beta oligomers.
Keywords: Alzheimer's disease; autopsy; catecholamines; dopamine; human; locus coeruleus; neuropathology; noradrenaline; substantia nigra; tauopathy; transcriptomics.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
A.J.E. has no relevant competing financial interests with this manuscript. A.J.E. serves as an unpaid steering committee member for the Genomic Answers for Children's Health Alliance at Leavitt Partners and as an unpaid executive committee member for the Neuromodulatory Subcortical System's Professional Interests Area of the International Society to Advance Alzheimer's Research and Treatment. S.T. has no relevant competing interests with this manuscript, but is co‐founder of Luxa Biotech developing an RPE therapy for AMD and has patents related to RPE cell therapy: Retinal pigment epithelial stem cells, Patent number: 8481313; Methods of treating a retinal disease by Retinal pigment epithelial stem cells, Patent number: 10034916; S.T. has advised BlueRock Therapeutics, Vita Therapeutics, and SANA Biotechnology. All other authors have no declared conflicts of interest to declare. Author disclosures are available in the Supporting Information.
Figures



References
-
- Rub U, Stratmann K, Heinsen H, et al. The brainstem tau cytoskeletal pathology of Alzheimer's disease: a brief historical overview and description of its anatomical distribution pattern, evolutional features, pathogenetic and clinical relevance. Curr Alzheimer Res. 2016;13(10):1178‐1197. doi:10.2174/1567205013666160606100509 - DOI - PubMed
MeSH terms
Substances
Grants and funding
- Rainwater Charitable Foundation
- P30 AG062422/NH/NIH HHS/United States
- T32 GM139780/NH/NIH HHS/United States
- K24AG053435/NH/NIH HHS/United States
- R01AG064314/NH/NIH HHS/United States
- R01AG060477/NH/NIH HHS/United States
- R01AG075802/NH/NIH HHS/United States
- UC Berkeley Greater Good Science Center
- 06/55318/Fundacao de Apoio a Pesquisa do Estado de São Paulo
- 2009/09134-4/Fundacao de Apoio a Pesquisa do Estado de São Paulo
- 2012/07526-5/Fundacao de Apoio a Pesquisa do Estado de São Paulo
- AARG-20-678884/ALZ/Alzheimer's Association/United States
- UC Berkeley Neuro-AI Resilience Center
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

