Sox9 in the second heart field and the development of the outflow tract; implications for cardiac septation and valve formation
- PMID: 40135884
- PMCID: PMC12380224
- DOI: 10.1002/dvdy.70014
Sox9 in the second heart field and the development of the outflow tract; implications for cardiac septation and valve formation
Abstract
Background: Previously, we explored the role of Sox9 in the second heart field (SHF) in atrioventricular septation. For that study, we created a SHF-specific Sox9 knockout mouse. In addition to the presence of primary atrial septal defects in half of the offspring, we found that virtually all specimens also developed a ventricular septal defect. Histological analysis suggested that the ventricular septal defects resulted from developmental perturbation of the mesenchymal structures within the outflow tract. In the current study, we investigated the role of Sox9 in the SHF in the development of these tissues.
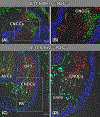
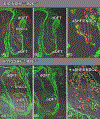
Results: Sox9 is expressed in all mesenchymal cell populations in the developing outflow tract, including a cohort of endocardial-derived cells that originate from the SHF-derived endocardium. SHF-specific deletion of Sox9 inhibits the formation of this cell population and ultimately leads to truncation of the mesenchymal outlet septum. This prevents complete fusion of this outlet septum with the atrioventricular mesenchymal complex, resulting in ventricular septal defects.
Conclusions: In combination with our first paper on the role of Sox9 in atrioventricular septation, data presented in this study demonstrate that Sox9 expression in the SHF is of critical importance for the proper formation of the septal structures in the developing heart.
Keywords: cell lineages; defects; development; heart; sox transcription factors.
© 2025 American Association for Anatomy.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
Authors have no conflict of interest to disclose. All experiments were approved by the MUSC Institutional Animal Care and Use Committee and complied with all federal and institutional guidelines.
Figures













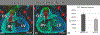

References
-
- Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1(3):435–440. S1534–5807(01)00040–5 [pii]. - PubMed
-
- Verzi MP, McCulley DJ, De Val S, Dodou E, Black BL. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol. 2005;287(1):134–145. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

