Abortive PDCoV infection triggers Wnt/β-catenin pathway activation, enhancing intestinal stem cell self-renewal and promoting chicken resistance
- PMID: 40135895
- PMCID: PMC11998530
- DOI: 10.1128/jvi.00137-25
Abortive PDCoV infection triggers Wnt/β-catenin pathway activation, enhancing intestinal stem cell self-renewal and promoting chicken resistance
Abstract
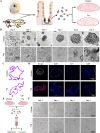
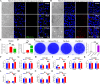
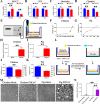
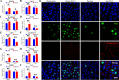
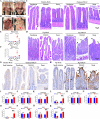
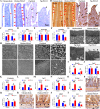
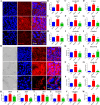
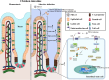
Porcine deltacoronavirus (PDCoV) is an emerging coronavirus causing economic losses to swine industries worldwide. PDCoV can infect chickens under laboratory conditions, usually with no symptoms or mild symptoms, and may cause outbreaks in backyard poultry and wildfowl, posing a potential risk of significant economic loss to the commercial poultry industry. However, the reasons for such a subdued reaction after infection are not known. Here, using chicken intestinal organoid monolayers, we found that although PDCoV infects them nearly as well as porcine intestinal organoid monolayers, infection did not result in detectable amounts of progeny virus. In ex vivo and in vivo experiments using chickens, PDCoV infection failed to initiate interferon and inflammatory responses. Additionally, infection did not result in a disrupted intestinal barrier nor a reduced number of goblet cells and mucus secretion, as in pigs. In fact, the number of goblet cells increased as did the secreted mucus, thereby providing an enhanced protective barrier. Ex vivo PDCoV infection in chicken triggered activation of the Wnt/β-catenin pathway with the upregulation of Wnt/β-catenin pathway genes (Wnt3a, Lrp5, β-catenin, and TCF4) and Wnt target genes (Lgr5, cyclin D1, and C-myc). This activation stimulates the self-renewal of intestinal stem cells (ISCs), accelerating ISC-mediated epithelial regeneration by significant up-regulation of PCNA (transiently amplifying cells), BMI1 (ISCs), and Lyz (Paneth cells). Our data demonstrate that abortive infection of PDCoV in chicken cells activates the Wnt/β-catenin pathway, which facilitates the self-renewal and proliferation of ISCs, contributing to chickens' resistance to PDCoV infection.IMPORTANCEThe intestinal epithelium is the main target of PDCoV infection and serves as a physical barrier against pathogens. Additionally, ISCs are charged with tissue repair after injury, and promoting rapid self-renewal of intestinal epithelium will help to re-establish the physical barrier and maintain intestinal health. We found that PDCoV infection in chicken intestinal organoid monolayers resulted in abortive infection and failed to produce infectious virions, disrupt the intestinal barrier, reduce the number of goblet cells and mucus secretion, and induce innate immunity, but rather increased goblet cell numbers and mucus secretion. Abortive PDCoV infection activated the Wnt/β-catenin pathway, enhancing ISC renewal and accelerating the renewal and replenishment of shed PDCoV-infected intestinal epithelial cells, thereby enhancing chicken resistance to PDCoV infection. This study provides novel insights into the mechanisms underlying the mild or asymptomatic response to PDCoV infection in chickens, which is critical for understanding the virus's potential risks to the poultry industry.
Keywords: PDCoV; Wnt/β-catenin signaling pathway; abortive infection; chicken; intestinal barrier; intestinal organoids; intestinal stem cells; pig.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Transmissible Gastroenteritis Virus Infection Promotes the Self-Renewal of Porcine Intestinal Stem Cells via Wnt/β-Catenin Pathway.J Virol. 2022 Sep 28;96(18):e0096222. doi: 10.1128/jvi.00962-22. Epub 2022 Sep 8. J Virol. 2022. PMID: 36073923 Free PMC article.
-
Porcine Deltacoronavirus Infection Disrupts the Intestinal Mucosal Barrier and Inhibits Intestinal Stem Cell Differentiation to Goblet Cells via the Notch Signaling Pathway.J Virol. 2023 Jun 29;97(6):e0068923. doi: 10.1128/jvi.00689-23. Epub 2023 Jun 8. J Virol. 2023. PMID: 37289083 Free PMC article.
-
Notoginsenoside R1 promotes Lgr5+ stem cell and epithelium renovation in colitis mice via activating Wnt/β-Catenin signaling.Acta Pharmacol Sin. 2024 Jul;45(7):1451-1465. doi: 10.1038/s41401-024-01250-7. Epub 2024 Mar 15. Acta Pharmacol Sin. 2024. PMID: 38491161 Free PMC article.
-
Roles of nAChR and Wnt signaling in intestinal stem cell function and inflammation.Int Immunopharmacol. 2020 Apr;81:106260. doi: 10.1016/j.intimp.2020.106260. Epub 2020 Jan 30. Int Immunopharmacol. 2020. PMID: 32007796 Review.
-
Molecular regulation mechanism of intestinal stem cells in mucosal injury and repair in ulcerative colitis.World J Gastroenterol. 2023 Apr 28;29(16):2380-2396. doi: 10.3748/wjg.v29.i16.2380. World J Gastroenterol. 2023. PMID: 37179583 Free PMC article. Review.
References
-
- Anonymous . 2012. Family - Coronaviridae, p 806–828. In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (ed), Virus Taxonomy. Elsevier, San Diego.
-
- Woo PCY, Lau SKP, Lam CSF, Lau CCY, Tsang AKL, Lau JHN, Bai R, Teng JLL, Tsang CCC, Wang M, Zheng B-J, Chan K-H, Yuen K-Y. 2012. Discovery of seven novel mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J Virol 86:3995–4008. doi:10.1128/JVI.06540-11 - DOI - PMC - PubMed
-
- Li W, Hulswit RJG, Kenney SP, Widjaja I, Jung K, Alhamo MA, van Dieren B, van Kuppeveld FJM, Saif LJ, Bosch B-J. 2018. Broad receptor engagement of an emerging global coronavirus may potentiate its diverse cross-species transmissibility. Proc Natl Acad Sci USA 115. doi:10.1073/pnas.1802879115 - DOI - PMC - PubMed
-
- Wu F, Zhao S, Yu B, Chen Y-M, Wang W, Song Z-G, Hu Y, Tao Z-W, Tian J-H, Pei Y-Y, Yuan M-L, Zhang Y-L, Dai F-H, Liu Y, Wang Q-M, Zheng J-J, Xu L, Holmes EC, Zhang Y-Z. 2020. A new coronavirus associated with human respiratory disease in China. Nature New Biol 579:265–269. doi:10.1038/s41586-020-2008-3 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 2023YFF1000900/National Key Research and Development Program of China
- BK20220581/Natural Science Foundation of Jiangsu Province
- 32402729/National Science Foundation For Young Scientists of China
- KYCX24_3805/Graduate Research and Innovation Projects of Jiangsu Province
- NA/Priority Academic Program Development of Jiangsu Higher Education Institutions
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

