RIG-I and cGAS mediate antimicrobial and inflammatory responses of primary osteoblasts and osteoclasts to Staphylococcus aureus
- PMID: 40135931
- PMCID: PMC12077190
- DOI: 10.1128/mbio.03971-24
RIG-I and cGAS mediate antimicrobial and inflammatory responses of primary osteoblasts and osteoclasts to Staphylococcus aureus
Abstract
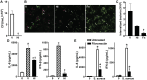
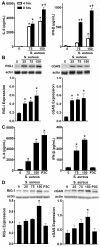
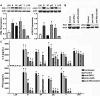
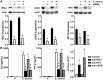
Staphylococcus aureus is the primary causative agent of osteomyelitis, and it is now apparent that osteoblasts and osteoclasts play a significant role in the pathogenesis of such infections. Their responses can either be protective or exacerbate inflammatory bone loss and are mediated by the recognition of microbial motifs by various pattern recognition receptors. We have recently reported that osteoblasts can respond to S. aureus challenge with the production of the type I interferon, interferon-beta, which can reduce the number of viable bacteria harbored within infected cells. In the present study, we demonstrate that S. aureus viability and internalization are necessary for maximal inflammatory cytokine and type I interferon responses of primary bone cells to this pathogen. Importantly, we show that primary murine and human bone cells constitutively express the cytosolic nucleic acid sensors, retinoic acid inducible gene I (RIG-I) and cyclic GMP-AMP synthase (cGAS), and demonstrate that such expression is markedly upregulated following S. aureus infection. The functional status of RIG-I and cGAS in osteoblasts and osteoclasts was confirmed by showing that specific ligands for each can also elevate their expression and induce cytokine responses. We have verified the specificity of such responses using siRNA knockdown or pharmacological inhibition and used these approaches to demonstrate that both sensors play a pivotal role in bone cell responses to infection with clinically relevant strains of S. aureus. Finally, we have begun to establish the biological significance of RIG-I- and cGAS-mediated bone cell responses with the demonstration that their attenuation increases S. aureus burden in infected cells, suggesting a potentially protective role for these sensors in osteomyelitis.IMPORTANCEStaphylococcal osteomyelitis is a severe infection that is often recalcitrant to current treatment strategies. We and others have demonstrated that resident bone cells are not merely passive victims but can respond to bacteria with the production of an array of immune mediators, including type I interferons, that could serve to limit such infections. Here, we demonstrate the functional expression of two cytosolic nucleic acid sensors, retinoic acid inducible gene I and cyclic GMP-AMP synthase, in primary murine and human osteoblasts and murine osteoclasts. We show that these pattern recognition receptors mediate potentially protective bone cell type I interferon responses to Staphylococcus aureus infection.
Keywords: RIG-I; Staphylococcus aureus; cGAS; cytokines; osteoblasts; osteoclasts; type I interferon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
