Kinetic Studies Reveal that the Secondary Station Impacts the Rate of Motion of Cyclobis(Paraquat-p-Phenylene) in Out-of-Equilibrium [2]Rotaxanes
- PMID: 40136063
- PMCID: PMC12143453
- DOI: 10.1002/cplu.202500154
Kinetic Studies Reveal that the Secondary Station Impacts the Rate of Motion of Cyclobis(Paraquat-p-Phenylene) in Out-of-Equilibrium [2]Rotaxanes
Abstract
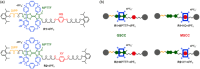
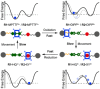
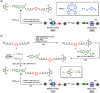
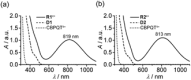
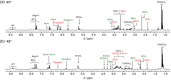
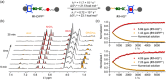
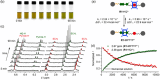
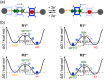
Control of movement in artificial molecular machines relies on the formation of out-of-equilibrium states that can subsequently interconvert to their ground states. However, a detailed description of molecular machines that are out of equilibrium is a challenge because they are often too short-lived to be characterized. Herein, the synthesis of two cyclobis(paraquat-p-phenylene) [2]rotaxanes that incorporate a redox-active monopyrrolotetrathiafulvalene unit as the primary station and either a hydroquinone or a xylyl moiety as the secondary station is described. It is shown that the bistable [2]rotaxanes can be pushed out of equilibrium by an oxidation/reduction cycle and since a steric barrier is located between the two stations, the out-of-equilibrium states of the [2]rotaxanes can be physically isolated as solids. This allows to make detailed spectroscopic and electrochemical investigations of the [2]rotaxanes in both the di-oxidized and un-oxidized states. The outcome of the studies shows that the replacement of the secondary hydroquinone station with a xylyl station has no impact on the thermodynamic properties but has a significant effect on the kinetic properties of the [2]rotaxanes illustrating that the nature of the secondary station can be used to control the speed of [2]rotaxane-based molecular machines.
Keywords: kinetics; molecular machines; out‐of‐equilibrium states; rotaxanes; tetrathiafulvalenes.
© 2025 The Author(s). ChemPlusChem published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Giuseppone N., Walther A., in Out‐of‐Equilibrium (Supra)molecular Systems and Materials, Wiley‐VCH, Hoboken, NJ: 2021.
-
- Seale J. S. W., Feng Y., Feng L., Astumian R. D., Stoddart J. F., Chem. Soc. Rev. 2022, 51, 8450. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

