Quantitative Bias Analysis for Single-Arm Trials With External Control Arms
- PMID: 40136297
- PMCID: PMC11947839
- DOI: 10.1001/jamanetworkopen.2025.2152
Quantitative Bias Analysis for Single-Arm Trials With External Control Arms
Abstract
Importance: Unmeasured confounding is a key concern for decision-makers when observational datasets are used to assemble external control arms (ECAs) for single-arm trials.
Objective: To investigate the utility of quantitative bias analysis (QBA) for exploring the sensitivity to unmeasured confounding of nonrandomized analyses using ECAs.
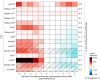
Design, setting, and participants: This study emulated 15 treatment comparisons using experimental arms from existing randomized trials in advanced non-small cell lung cancer (aNSCLC) conducted after 2011 and ECAs derived from observational data. Participants were eligible individuals diagnosed with aNSCLC between January 1, 2011, and March 1, 2020. After adjustment for measured baseline confounders, a prespecified QBA was conducted to address potential bias by known unmeasured and mismeasured confounders. The QBA relied on a synthesis of external evidence from a targeted literature search, randomized trial data, and clinician input. Hazard ratios from the original randomized trials were compared with those from their emulation based on ECA analyses. Analyses were completed from February 2022 to October 2023.
Exposure: Initiation of systemic therapies for aNSCLC.
Main outcomes and measures: Hazard ratios for all-cause death.
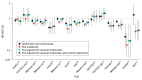
Results: Sample sizes varied from 52 to 830 depending on the treatment group. The mean difference in the log hazard ratio estimates when using the original control arm vs the ECA for each trial was 0.247 in unadjusted analyses (ratio of hazard ratios, 1.36), 0.139 when adjusted for measured confounders (ratio of hazard ratios, 1.22), and 0.098 when adding external adjustment for unmeasured and mismeasured confounders (ratio of hazard ratios, 1.17).
Conclusions and relevance: QBA was feasible and informative in ECA analyses in which residual confounding was expected to be the most important source of bias. These findings encourage further exploration of how QBA can help quantify the impact of bias in other settings and when using other data sources.
Conflict of interest statement
Figures
References
-
- Lash TL, Fink AK. Applying Quantitative Bias Analysis to Epidemiologic Data. Springer; 2009. doi:10.1007/978-0-387-87959-8 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

