Factors in Time to Full Approval or Withdrawal for Anticancer Medicines Granted Accelerated Approval by the FDA
- PMID: 40136298
- PMCID: PMC11947834
- DOI: 10.1001/jamanetworkopen.2025.2026
Factors in Time to Full Approval or Withdrawal for Anticancer Medicines Granted Accelerated Approval by the FDA
Abstract
Importance: The accelerated approval pathway was developed to expedite US Food and Drug Administration (FDA) drug approval for life-threatening conditions based on changes to unvalidated surrogate measures, with conversion to regular approval then based on a required confirmatory trial. However, confirmatory trials may not be completed in a timely fashion.
Objective: To analyze factors associated with time to conversion to regular approval.
Design, setting, and participants: This cohort study of cancer drugs FDA-approved from 1992 to 2022 extracted pivotal and confirmatory trial characteristics, outcomes, safety data, and confirmatory study status at approval from drug labels and published reports. Clinical benefit was assessed using the European Society of Medical Oncology-Magnitude of Clinical Benefit Scale (ESMO-MCBS) for pivotal and confirmatory studies. High benefit was defined as grade A or B (for trials of curative intent) and 4 or 5 (palliative intent) and low benefit as grade C and 2 or below. Data were analyzed between August 2023 and August 2024.
Main outcome and measure: Time to full approval or withdrawal.
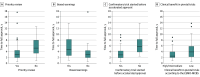
Results: This cohort study analyzed 102 cancer drug indications granted accelerated approval by the FDA between 1992 and 2022 and converted to regular approval by August 31, 2024. The median (IQR) time to conversion was 3.1 (1.9-4.8) years. Of these, 83 (81%) received priority review, and 27 (26%) carried boxed warnings. On the ESMO-MCBS scale, 12 of 101 pivotal trials that were scorable showed high benefit (12%), 27 intermediate benefit (27%), and 62 low benefit (61%). Twenty-one confirmatory trials (21%) were initiated after accelerated approval. Factors at the time of accelerated approval that were associated with shorter times to full approval included priority review designation, absence of boxed warnings, initiation of confirmatory studies before approval, and pivotal trials showing intermediate or high benefit on the ESMO-MCBS framework. Among the 102 confirmatory trials, 34 (33%) demonstrated significant improvements in overall survival, and 14 of 35 trials (40%) reporting on quality of life showed significant benefits. The ESMO-MCBS scale was applicable to 98 trials, with 46 (47%) scoring high clinical benefit, 29 (30%) intermediate, and 23 (23%) low. Indications with benefits in overall survival (median [IQR], 2.15 [1.40-3.38] vs 3.70 [2.33-5.78] years), quality of life (median [IQR], 2.29 [1.85-3.53] vs 4.22 [2.52-5.72] years), and high clinical benefit in confirmatory trials (median [IQR], 2.34 [1.52-3.39] vs 3.91 [2.59-6.42] years) were associated with faster conversion to full approval.
Conclusions and relevance: In this cohort study, drugs with low clinical benefit or safety concerns at accelerated approval were linked to delayed confirmatory studies, while high clinical benefit in confirmatory trials correlated with faster conversion. These associations can help guide patient decision-making around accelerated approval drugs.
Conflict of interest statement
Figures

Comment in
- doi: 10.1001/jamanetworkopen.2025.2040
References
-
- National Archives . 21 Code of Federal Regulations, Part 314.510. Accessed February 27, 2025. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-D/part-314/su...
-
- National Archives . 21 Code of Federal Regulations, Part 601.41. Accessed February 27, 2025. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-F/part-601/su...
-
- National Archives . 21 Code of Federal Regulations, Part 314.530. Accessed February 27, 2025. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-D/part-314/su...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

