Evaluation of Clinical Parameters Associated with Response and Resistance to Cemiplimab in Locally Advanced and Metastatic Cutaneous Squamous Cell Carcinoma: A Multi-Institutional Retrospective Cohort Study
- PMID: 40136372
- PMCID: PMC11941019
- DOI: 10.3390/curroncol32030168
Evaluation of Clinical Parameters Associated with Response and Resistance to Cemiplimab in Locally Advanced and Metastatic Cutaneous Squamous Cell Carcinoma: A Multi-Institutional Retrospective Cohort Study
Abstract
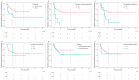
Cutaneous squamous cell carcinoma (cSCC) is a common cancer with increasing incidence and 5% of patients develop incurable disease, often resistant to chemotherapy. The anti-PD-1 therapy cemiplimab has shown high efficacy in clinical trials. This retrospective study evaluated the real-world effectiveness of cemiplimab in incurable cSCC and examined factors influencing response and toxicity. Data from 86 patients across three UK healthcare providers were analysed. Median progression-free survival (PFS) and overall survival (OS) were not reached, with 38% showing durable responses beyond 12 months. The overall response rate was 60.8% (95% CI 49-71), and the clinical benefit rate was 74.3% (95% CI 63-83). A head and neck primary site was associated with improved PFS (p = 0.008) and OS (p = 0.023), while concurrent immunosuppression was associated with worse PFS (p < 0.001). These findings align with clinical trials, suggesting cemiplimab is effective and safe in the recurrent/metastatic setting.
Keywords: cemiplimab; cutaneous squamous cell carcinoma; primary site.
Conflict of interest statement
R.M. reports the following: Honoraria: Bristol Myers Squibb (BMS), Merck Sharp and Dohme (MSD), Roche, Bayer, Achilles Therapeutics, Aptus Clinical, PCI Biotech, Ayala Pharmaceuticals, Oxsonics. O.D. reports the following: Honoraria: Sanofi, Regeneron, Achilles Therapeutics; Speaking fees: Sanofi, Regeneron. G.F. reports the following: Consulting or Advisory Role: Bristol Myers Squibb (BMS), Janssen, Novartis, Pfizer, Eisai; Speakers’ Bureau: Roche, Bristol Myers Squibb (BMS), Novartis, Eisai, Pierre Fabre, Merck Serono, Janssen Oncology, MSD Oncology, Sanofi, Astellas Pharma, Pfizer, Bayer; Travel, Accommodations, Expenses: Novartis, Janssen, Bayer, Merck Serono, Recordati. The other authors have no conflicts of interest to declare.
Figures


References
-
- New Data Shows a Record 224,000 Skin Cancers in England in 2019. [(accessed on 12 September 2023)]. Available online: https://www.skinhealthinfo.org.uk/new-data-shows-a-record-224000-skin-ca....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

