The Use of Heterologous Antigens for Biopanning Enables the Selection of Broadly Neutralizing Nanobodies Against SARS-CoV-2
- PMID: 40136472
- PMCID: PMC11939171
- DOI: 10.3390/antib14010023
The Use of Heterologous Antigens for Biopanning Enables the Selection of Broadly Neutralizing Nanobodies Against SARS-CoV-2
Abstract
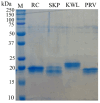
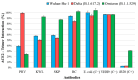
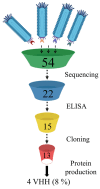
Background: Since the emergence of SARS-CoV-2 in the human population, the virus genome has undergone numerous mutations, enabling it to enhance transmissibility and evade acquired immunity. As a result of these mutations, most monoclonal neutralizing antibodies have lost their efficacy, as they are unable to neutralize new variants. Antibodies that neutralize a broad range of SARS-CoV-2 variants are of significant value in combating both current and potential future variants, making the identification and development of such antibodies an ongoing critical goal. This study discusses the strategy of using heterologous antigens in biopanning rounds. Methods: After four rounds of biopanning, nanobody variants were selected from a phage display library. Immunochemical methods were used to evaluate their specificity to the S protein of various SARS-CoV-2 variants, as well as to determine their competitive ability against ACE2. Viral neutralization activity was analyzed. A three-dimensional model of nanobody interaction with RBD was constructed. Results: Four nanobodies were obtained that specifically bind to the receptor-binding domain (RBD) of the SARS-CoV-2 spike glycoprotein and exhibit neutralizing activity against various SARS-CoV-2 strains. Conclusions: The study demonstrates that performing several rounds of biopanning with heterologous antigens allows the selection of nanobodies with a broad reactivity spectrum. However, the fourth round of biopanning does not lead to the identification of nanobodies with improved characteristics.
Keywords: AlphaFold; SARS-CoV-2; VHH; affinity selection; biopanning; molecular dynamics; nanobodies; phage library; protein–protein docking; recombinant antibodies; single-domain antibodies.
Conflict of interest statement
The authors declare no conflicts of financial/non-financial interests related to the writing of this article.
Figures

Similar articles
-
Isolation of a human monoclonal antibody specific for the receptor binding domain of SARS-CoV-2 using a competitive phage biopanning strategy.Antib Ther. 2020 Apr 30;3(2):95-100. doi: 10.1093/abt/tbaa008. eCollection 2020 Apr. Antib Ther. 2020. PMID: 33912790 Free PMC article.
-
Deep mutational engineering of broadly-neutralizing nanobodies accommodating SARS-CoV-1 and 2 antigenic drift.MAbs. 2022 Jan-Dec;14(1):2076775. doi: 10.1080/19420862.2022.2076775. MAbs. 2022. PMID: 35593235 Free PMC article.
-
A Competitive Panning Method Reveals an Anti-SARS-CoV-2 Nanobody Specific for an RBD-ACE2 Binding Site.Vaccines (Basel). 2023 Feb 6;11(2):371. doi: 10.3390/vaccines11020371. Vaccines (Basel). 2023. PMID: 36851249 Free PMC article.
-
Structural and Computational Studies of the SARS-CoV-2 Spike Protein Binding Mechanisms with Nanobodies: From Structure and Dynamics to Avidity-Driven Nanobody Engineering.Int J Mol Sci. 2022 Mar 8;23(6):2928. doi: 10.3390/ijms23062928. Int J Mol Sci. 2022. PMID: 35328351 Free PMC article. Review.
-
The Biological Functions and Clinical Significance of SARS-CoV-2 Variants of Corcern.Front Med (Lausanne). 2022 May 20;9:849217. doi: 10.3389/fmed.2022.849217. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35669924 Free PMC article. Review.
References
-
- Barbieri E.S., Sosa-Holt C., Ibañez L.I., Baztarrica J., Garaicoechea L., Gay C.L., Caceres C.J., Aduriz M., Baumeister E., Escribano J.A., et al. Anti-hemagglutinin monomeric nanobody provides prophylactic immunity against H1 subtype influenza A viruses. PLoS ONE. 2024;19:e0301664. doi: 10.1371/journal.pone.0301664. - DOI - PMC - PubMed
-
- Esmagambetov I.B., Shcheblyakov D.V., Egorova D.A., Voronina O.L., Derkaev A.A., Voronina D.V., Popova O., Ryabova E.I., Shcherbinin D.N., Aksenova E.I., et al. Nanobodies Are Potential Therapeutic Agents for the Ebola Virus Infection. Acta Naturae. 2021;13:53–63. doi: 10.32607/actanaturae.11487. - DOI - PMC - PubMed
-
- Hruškovicová J., Bhide K., Petroušková P., Tkáčová Z., Mochnáčová E., Čurlík J., Bhide M., Kulkarni A. Engineering the Single Domain Antibodies Targeting Receptor Binding Motifs Within the Domain III of West Nile Virus Envelope Glycoprotein. Front. Microbiol. 2022;13:801466. doi: 10.3389/fmicb.2022.801466. - DOI - PMC - PubMed
-
- Llauger G., Monti D., Adúriz M., Romão E., Dumón A.D., Mattio M.F., Wigdorovitz A., Muyldermans S., Vincke C., Parreño V., et al. Development of Nanobodies against Mal de Río Cuarto virus major viroplasm protein P9-1 for diagnostic sandwich ELISA and immunodetection. Sci. Rep. 2021;11:20013. doi: 10.1038/s41598-021-99275-z. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

