Mutual Interactions Between Microbiota and the Human Immune System During the First 1000 Days of Life
- PMID: 40136555
- PMCID: PMC11940030
- DOI: 10.3390/biology14030299
Mutual Interactions Between Microbiota and the Human Immune System During the First 1000 Days of Life
Abstract
The development of the human immune system starts during the fetal period in a largely, but probably not completely, sterile environment. During and after birth, the immune system is exposed to an increasingly complex microbiota. The first microbiota encountered during passage through the birth canal colonize the infant gut and induce the tolerance of the immune system. Transplacentally derived maternal IgG as well as IgA from breast milk protect the infant from infections during the first 100 days, during which the immune system further develops and immunological memory is formed. The Weaning and introduction of solid food expose the immune system to novel (food) antigens and allow for other microbiota to colonize. The cells and molecules involved in the mutual and intricate interactions between microbiota and the developing immune system are now beginning to be recognized. These include bacterial components such as polysaccharide A from Bacteroides fragilis, as well as bacterial metabolites such as the short-chain fatty acid butyrate, indole-3-aldehyde, and indole-3-propionic acid. All these, and probably more, bacterial metabolites have specific immunoregulatory functions which shape the development of the human immune system during the first 1000 days of life.
Keywords: bacterial colonization; gut microbiota; mode of delivery; neonatal immune system; prenatal delivery; short-chain fatty acids (SCFAs).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

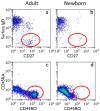


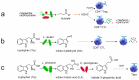

References
-
- Cupedo T., Crellin N.K., Papazian N., Rombouts E.J., Weijer K., Grogan J.L., Fibbe W.E., Cornelissen J.J., Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat. Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

