Clinical Efficacy of Prolotherapy for Temporomandibular Joint Disorders: A Systematic Review and Meta-Analysis
- PMID: 40136587
- PMCID: PMC11941112
- DOI: 10.3390/clinpract15030051
Clinical Efficacy of Prolotherapy for Temporomandibular Joint Disorders: A Systematic Review and Meta-Analysis
Abstract
Background: Temporomandibular disorders (TMDs) encompass a group of conditions characterized by anatomical, histological, and/or functional abnormalities that affect the muscular and/or articular components of the temporomandibular joint. Prolotherapy is an injectable treatment modality for chronic musculoskeletal pain that involves dextrose solution administration in the joint. Aims: To summarize, the aims involve considering the existing quality of clinical evidence on the efficacy of prolotherapy versus placebo and other active comparators, such as autologous blood products or botulinum toxin, in improving the outcomes of TMDs. Methods: A literature search in MEDLINE, Scopus, and Cochrane databases was performed, following the PRISMA statement guidelines, to identify randomized controlled trials (RCTs) of patients with TMDs receiving prolotherapy. The maximal incisor opening (MIO), visual analogue score (VAS) for pain, and frequency of dislocations were analyzed as the outcomes. The weighted mean difference was used to pool outcomes. The risk of bias was recorded for the included studies. Results: Six studies comparing prolotherapy to placebo were identified. Prolotherapy is uniformly more efficient in reducing the VAS for pain when compared to the placebo (mean difference = 1.20, 95%CI: 0.56-1.84, p < 0.001). Perceived jaw mobility was improved among prolotherapy patients, (mean difference = 0.47, 95%CI: 0.05-0.90, p = 0.003) when compared to the placebo. A beneficial effect for prolotherapy with regard to MIO (mean difference = 0.84, 95%CI: -2.12-3.80, p = 0.58) was not confirmed. Prolotherapy appears to be more efficient than autologous blood products in reducing VAS for pain (mean difference = 0.49, 95%CI: 0.11-0.87, p = 0.01). Prolotherapy was found to be more effective in reducing pain, MIO, and clicking when compared to an occlusal splint in a single study. Conclusions: Prolotherapy is also a promising modality for TMDs, despite the limited number of randomized clinical trials. Existing evidence supports its use to reduce TMD-related pain, even against other modalities. Further research is needed to better describe the benefit of prolotherapy for other outcomes.
Keywords: meta-analysis; placebo; prolotherapy; randomized controlled trials; systematic review; temporomandibular disorder; temporomandibular joint.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

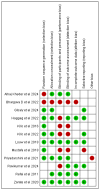

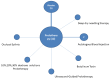



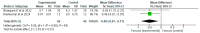
References
-
- Matheson E.M., Fermo J.D., Blackwelder R.S. Temporomandibular Disorders: Rapid Evidence Review. Am. Fam. Physician. 2023;107:52–58. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

