International Survey on Phenylketonuria Newborn Screening
- PMID: 40136633
- PMCID: PMC11943362
- DOI: 10.3390/ijns11010018
International Survey on Phenylketonuria Newborn Screening
Abstract
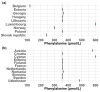
Newborn screening for Phenylketonuria enables early detection and timely treatment with a phenylalanine-restricted diet to prevent severe neurological impairment. Although effective and in use for 60 years, screening, diagnostic, and treatment practices still vary widely across countries and centers. To evaluate the Phenylketonuria newborn screening practices internationally, we designed a survey with questions focusing on the laboratory aspect of the screening system. We analyzed 24 completed surveys from 23 countries. Most participants used the same sampling age range of 48-72 h; they used tandem mass spectrometry and commercial non-derivatized kits to measure phenylalanine (Phe), and had non-negative cut-off values (COV) set mostly at 120 µmol/L of Phe. Participants mostly used genetic analysis of blood and detailed amino acid analysis from blood plasma as their confirmatory methods and set the COV for the initiation of dietary therapy at 360 µmol/L of Phe. There were striking differences in practice as well. While most participants reported a 48-72 h range for age at sampling, that range was overall quite diverse Screening COV varied as well. Additional screening parameters, e.g., the phenylalanine/tyrosine ratio were used by some participants to determine the screening result. Some participants included testing for tetrahydrobiopterin deficiency, or galactosemia in their diagnostic process. Results together showed that there is room to select a best practice from the many practices applied. Such a best practice of PKU-NBS parameters and post-screening parameters could then serve as a generally applicable guideline.
Keywords: cut-off; international; laboratory; methods; neonatal; newborn; phenylketonuria; screening; survey.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Fölling A. Über Ausscheidung von Phenylbrenztraubensäure in Den Harn Als Stoffwechselanomalie in Verbindung Mit Imbezillität. Hoppe-Seyler’s Z. Für Physiol. Chem. 1934;227:169–181. doi: 10.1515/bchm2.1934.227.1-4.169. - DOI
-
- van Wegberg A.M.J., MacDonald A., Ahring K., Bélanger-Quintana A., Blau N., Bosch A.M., Burlina A., Campistol J., Feillet F., Giżewska M., et al. The Complete European Guidelines on Phenylketonuria: Diagnosis and Treatment. Orphanet J. Rare Dis. 2017;12:162. doi: 10.1186/s13023-017-0685-2. - DOI - PMC - PubMed
-
- Perko D., Groselj U., Cuk V., Iztok Remec Z., Zerjav Tansek M., Drole Torkar A., Krhin B., Bicek A., Oblak A., Battelino T., et al. Comparison of Tandem Mass Spectrometry and the Fluorometric Method—Parallel Phenylalanine Measurement on a Large Fresh Sample Series and Implications for Newborn Screening for Phenylketonuria. Int. J. Mol. Sci. 2023;24:2487. doi: 10.3390/ijms24032487. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

