Differential Regulation of PKM2, AMPK, and mTOR in Response to Insulin and Dietary Management
- PMID: 40136665
- PMCID: PMC11940920
- DOI: 10.3390/cells14060416
Differential Regulation of PKM2, AMPK, and mTOR in Response to Insulin and Dietary Management
Abstract
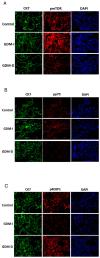
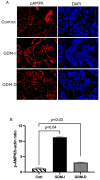
Gestational diabetes mellitus (GDM) affects placental metabolism, influencing both maternal and fetal outcomes. This study investigated the expression of metabolic regulators-Pyruvate Kinase M2 (PKM2), AMP-activated protein kinase (AMPK), and mTOR pathway components-in placental tissues from GDM pregnancies managed with either insulin (GDM-I) or dietary interventions (GDM-D). We hypothesize that metabolic adaptation in GDM is differentially regulated by treatment modality. This study analyzed 30 cases, including 10 control pregnancies,10 GDM-D cases, and 10 GDM-I cases. Analytical methods included immunofluorescence and immunoblotting. We observed an upregulation of PKM2 in both GDM-I and GDM-D placentas, suggesting enhanced glycolytic adaptation under GDM-induced metabolic stress. AMPK expression was significantly elevated in GDM-I and moderately increased in GDM-D placentas, potentially compensating for insulin resistance by promoting glucose uptake and energy homeostasis. Furthermore, mTOR pathway activation differed by treatment type, suggesting a treatment-specific mTOR response. The metabolic changes observed suggest that treatment modality in GDM may have direct implications for maternal and fetal health. Our findings indicate that while insulin and dietary management support metabolic adaptation in GDM, they do so through distinct mechanisms. These findings support a personalized approach in GDM treatment, where patient-specific metabolic responses should guide therapeutic decisions.
Keywords: AMPK; GDM; PKM2; mTOR; placental metabolism.
Conflict of interest statement
The authors declare they have no financial or non-financial conflicts of interest.
Figures



References
-
- Mejia C., Lewis J., Jordan C., Mejia J., Ogden C., Monson T., Winden D., Watson M., Reynolds P.R., Arroyo J.A. Decreased activation of placental mTOR family members is associated with the induction of intrauterine growth restriction by secondhand smoke in the mouse. Cell Tissue Res. 2017;367:387–395. doi: 10.1007/s00441-016-2496-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

