Lateral Ventricular Neural Stem Cells Provide Negative Feedback to Circuit Activation Through GABAergic Signaling
- PMID: 40136675
- PMCID: PMC11940892
- DOI: 10.3390/cells14060426
Lateral Ventricular Neural Stem Cells Provide Negative Feedback to Circuit Activation Through GABAergic Signaling
Abstract
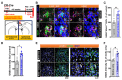
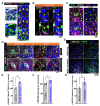
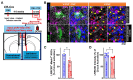
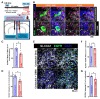
Recent studies have demonstrated that circuit activation in vivo can regulate proliferation of lateral ventricular neural stem cells (LV NSCs), although the underlying molecular and cellular mechanisms are not yet fully understood. Here, we investigated the role of GABAergic signaling in the interaction between LV NSCs and the anterior cingulate cortex-subependymal-choline acetyltransferase+ (ChAT+) neuron (ACC-subep-ChAT+) circuit. We found that monoamine oxidase B (MAOB), a key enzyme involved in gamma-aminobutyric acid (GABA) synthesis, is expressed in LV NSCs, and that activation of the ACC-subep-ChAT+ circuit can modulate MAOB activity. Additionally, LV NSCs express LRRC8D, a core component of volume-regulated anion channels, and GABA transporter-1 (GAT-1, SLC6A1). We show evidence that, through GABA signaling, LRRC8D and GAT-1 can provide a negative feedback signal to ChAT+ neurons, a key component of the ACC-subep-ChAT+ circuit that regulate proliferation of LV NSCs. These findings suggest that MAOB-driven GABA synthesis, LRRC8D-regulated chloride and GABA transport, and GAT-1-facilitated GABA reuptake can regulate neural circuit activation and influence NSC proliferation dynamics in the LV.
Keywords: GABA transporter-1 (GAT-1); GABAergic signaling; LRRC8D; SLC6A1; anterior cingulate cortex-subependymal-ChAT+ (ACC-subep-ChAT+) circuit; lateral ventricular neural stem cells (LV NSCs); monoamine oxidase B (MAOB); volume-regulated anion channels (VRACs).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
A cholinergic signaling pathway underlying cortical circuit activation of quiescent neural stem cells in the lateral ventricle.Sci Signal. 2024 Sep 24;17(855):eadk8810. doi: 10.1126/scisignal.adk8810. Epub 2024 Sep 24. Sci Signal. 2024. PMID: 39316665
-
Cortical regulation of neurogenesis and cell proliferation in the ventral subventricular zone.Cell Rep. 2023 Jul 25;42(7):112783. doi: 10.1016/j.celrep.2023.112783. Epub 2023 Jul 8. Cell Rep. 2023. PMID: 37422764 Free PMC article.
-
The relationship between GABA-containing cells and the cholinergic circuitry in the rabbit retina.Vis Neurosci. 2001 Jan-Feb;18(1):93-100. doi: 10.1017/s0952523801181083. Vis Neurosci. 2001. PMID: 11347820
-
Neural mechanisms underlying GABAergic regulation of adult hippocampal neurogenesis.Cell Tissue Res. 2018 Jan;371(1):33-46. doi: 10.1007/s00441-017-2668-y. Epub 2017 Sep 25. Cell Tissue Res. 2018. PMID: 28948349 Free PMC article. Review.
-
Developmental Formation of the GABAergic and Glycinergic Networks in the Mouse Spinal Cord.Int J Mol Sci. 2022 Jan 13;23(2):834. doi: 10.3390/ijms23020834. Int J Mol Sci. 2022. PMID: 35055019 Free PMC article. Review.
References
-
- de Sonnaville S., van Strien M.E., Middeldorp J., Sluijs J.A., van den Berge S.A., Moeton M., Donega V., van Berkel A., Deering T., De Filippis L., et al. The adult human subventricular zone: Partial ependymal coverage and proliferative capacity of cerebrospinal fluid. Brain Commun. 2020;2:fcaa150. doi: 10.1093/braincomms/fcaa150. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

