In Vitro Exposure to the Endocrine-Disrupting Chemical Climbazole Impairs Human Sperm Motility, Hormonal Signalling, and Mitochondrial Activity
- PMID: 40136676
- PMCID: PMC11940937
- DOI: 10.3390/cells14060427
In Vitro Exposure to the Endocrine-Disrupting Chemical Climbazole Impairs Human Sperm Motility, Hormonal Signalling, and Mitochondrial Activity
Abstract
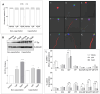
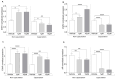
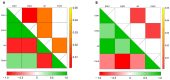
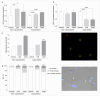
This study explores the endocrine-disrupting effects of climbazole (CBZ), an environmental and lifestyle stressor, on male fertility. The impact of CBZ on sperm vitality, motility, and molecular pathways related to hormone receptors and apoptosis was evaluated, in non-capacitated and capacitated conditions. Gene expression of key components, including hormone receptors (ESR1, ESR2, FSHR, AR), apoptosis-related genes (BAX, BCL2), and COX4l1 (involved in mitochondrial function), was analyzed. Protein tyrosine phosphorylation, a marker of capacitation, was also examined using immunofluorescence and Western blot analysis. We demonstrated that CBZ significantly reduced sperm vitality at concentrations above 25 µM and motility at 1 and 10 µM in non-capacitated and capacitated conditions. Changes in tyrosine phosphorylation patterns were also observed. Gene expression analysis revealed an upregulation of ESR1, ESR2, FSHR, and BAX, while AR and COX4l1 expression were downregulated. These findings offer new insights into the potential endocrine-disrupting and cytotoxic effects of CBZ, highlighting its potential role in compromising male reproductive health.
Keywords: climbazole (CBZ); endocrine-disrupting chemicals (EDCs); male fertility; oxidative stress; semen quality.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Luongo F.P., Annunzi E., Girolamo F., Belmonte G., Ponchia R., Piomboni P., Luddi A. Assessment of Sperm Chromosomal Abnormalities Using Fluorescence in Situ Hybridization (FISH): Implications for Reproductive Potential. J. Assist. Reprod. Genet. 2024;41:2787–2793. doi: 10.1007/s10815-024-03224-4. - DOI - PMC - PubMed
-
- Bergman Å., Heindel J.J., Jobling S., Kidd K.A., Zoeller R.T. State of the Science of Endocrine Disrupting Chemicals—2012: An Assessment of the State of the Science of Endocrine Disruptors Prepared by a Group of Experts for the United Nations Environment Programme (UNEP) and WHO. United National Environment Programme, World Health Organization; Geneva, Switzerland: 2012.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

