Chromosomal Instability Is Associated with cGAS-STING Activation in EGFR-TKI Refractory Non-Small-Cell Lung Cancer
- PMID: 40136696
- PMCID: PMC11941500
- DOI: 10.3390/cells14060447
Chromosomal Instability Is Associated with cGAS-STING Activation in EGFR-TKI Refractory Non-Small-Cell Lung Cancer
Abstract
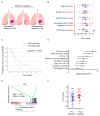
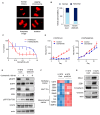
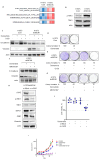
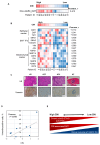
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) are standard therapies for EGFR-mutated non-small-cell lung cancer (NSCLC); however, their efficacy is inconsistent. Secondary mutations in the EGFR or other genes that lead to resistance have been identified, but resistance mechanisms have not been fully identified. Chromosomal instability (CIN) is a hallmark of cancer and results in genetic diversity. In this study, we demonstrated by transcriptomic analysis that CIN activates the cGAS-STING signaling pathway, which leads to EGFR-TKI refractoriness in a subset of EGFR-mutated NSCLC patients. Furthermore, EGFR-mutated H1975dnMCAK cells, which frequently underwent chromosomal mis-segregation, demonstrated refractoriness to the EGFR-TKI osimertinib compared to control cells. Second, H1975dnMCAK cells exhibited activation of cGAS-STING signaling and its downstream signaling, including tumor-promoting cytokine IL-6. Finally, chromosomally unstable EGFR-mutated NSCLC exhibited enhanced epithelial-mesenchymal transition (EMT). Blockade of cGAS-STING-TBK1 signaling reversed EMT, resulting in restored susceptibility to EGFR-TKIs in vitro and in vivo. These results suggest that CIN may lead to the activation of cGAS-STING signaling in some EGFR-mutated NSCLC, resulting in EMT-associated EGFR-TKI resistance.
Keywords: EGFR-mutated non-small-cell lung cancer; chromosomal instability; epidermal growth factor receptor tyrosine kinase inhibitors.
Conflict of interest statement
K.Y. and K.N. received research funding from Daiichi Sankyo Co., Ltd. Y.H., K.H., Y.K. and T.K. are employees of Daiichi Sankyo Co., Ltd. H.G., E.O., H.O. and M.F. are employees of Daiichi Sankyo RD Novare Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Mitsudomi T., Morita S., Yatabe Y., Negoro S., Okamoto I., Tsurutani J., Seto T., Satouchi M., Tada H., Hirashima T., et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. - DOI - PubMed
-
- Nakagawa K., Garon E.B., Seto T., Nishio M., Ponce Aix S., Paz-Ares L., Chiu C.H., Park K., Novello S., Nadal E., et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:1655–1669. doi: 10.1016/S1470-2045(19)30634-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

