Titanium Nitride as an Intermetallic Diffusion Barrier for Hydrogen Permeation in Palladium-Vanadium Composite Membranes
- PMID: 40137020
- PMCID: PMC11943908
- DOI: 10.3390/membranes15030068
Titanium Nitride as an Intermetallic Diffusion Barrier for Hydrogen Permeation in Palladium-Vanadium Composite Membranes
Abstract
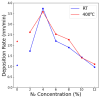
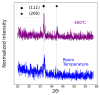
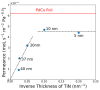
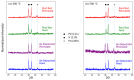
Hydrogen purification is a critical industrial process, and there are ongoing efforts to develop low-cost alternatives to palladium foil membranes. Titanium nitride (TiN) is studied as an interdiffusion barrier to enable hydrogen permeation in composite palladium-vanadium membranes. TiN was deposited via reactive sputtering, and films with the desired (200) orientation were obtained in the metallic regime at 400 °C under a 200 V bias to the substrate. The permeability of thin-film TiN was determined with palladium-based sandwich structures. TiN layers up to 10 nm resulted in a minimal decrease in flux (~20%) relative to a freestanding PdCu foil, which was attributed to the interfacial resistance. At greater thicknesses, the TiN layer was rate-limiting, and it was found that the effective permeability of the sputtered TiN thin films was ~6 × 10-12 mol s-1 m-1 Pa-0.5. Composite Pd|TiN|V|TiN|Pd membranes exhibited permeability values up to three times greater than pure palladium, exhibiting stability at 450 °C for over 100 h, with the lack of intermetallic diffusion and alloy formation being confirmed with XRD. The membranes were unstable at 500 °C, which was attributed to the instability of the thin Pd layer and loss of catalytic activity.
Keywords: diffusion barrier; hydrogen membranes; intermetallic diffusion; palladium; titanium nitride; vanadium.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Spillman R. Membrane Science and Technology. Volume 2. Elsevier; Amsterdam, The Netherlands: 1995. Chapter 13 Economics of Gas Separation Membrane Processes; pp. 589–667.
-
- McCarthy S., Braddock D.C., Wilton-Ely J.D.E.T. Strategies for Sustainable Palladium Catalysis. Coord. Chem. Rev. 2021;442:213925. doi: 10.1016/j.ccr.2021.213925. - DOI
-
- Fuerst T.F., Petsalis E.P., Wolden C.A., Way J.D. Application of TiC in Vanadium-Based Hydrogen Membranes. Ind. Eng. Chem. Res. 2018;57:16084–16094. doi: 10.1021/acs.iecr.8b02452. - DOI
-
- Job A.L., Li C., Fuerst T.F., Douglas Way J., Wolden C.A. Acceleration of Pd-V Intermetallic Diffusion by Hydrogen. J. Alloys Compd. 2024;972:172825. doi: 10.1016/j.jallcom.2023.172825. - DOI
-
- Fuerst T.F., Zhang Z., Hentges A.M., Lundin S.-T.B., Wolden C.A., Way J.D. Fabrication and Operational Considerations of Hydrogen Permeable Mo2C/V Metal Membranes and Improvement with Application of Pd. J. Membr. Sci. 2018;549:559–566. doi: 10.1016/j.memsci.2017.12.030. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

