Transcriptional Regulation Mechanisms in Adaptively Evolved Pichia kudriavzevii Under Acetic Acid Stress
- PMID: 40137215
- PMCID: PMC11942776
- DOI: 10.3390/jof11030177
Transcriptional Regulation Mechanisms in Adaptively Evolved Pichia kudriavzevii Under Acetic Acid Stress
Abstract
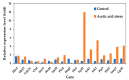
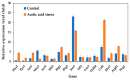
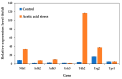
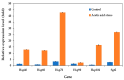
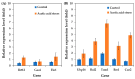
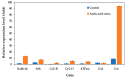
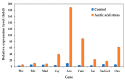
Acetic acid, a common weak acid in industrial fermentation processes, occurs naturally in lignocellulosic hydrolysates and is a byproduct of microbial metabolism. As a significant environmental stressor, it triggers the expression of multiple genes involved in various cellular responses, including biological processes, cellular components, and molecular functions. Using the acid-tolerant strain Pichia kudriavzevii PkAC-9, developed through adaptive laboratory evolution under acetic acid stress, we conducted a transcriptional analysis of 70 stress response-associated genes. RT-qPCR analysis revealed significant upregulation of several genes compared with the wild-type strain under acetic acid stress conditions. The most dramatic changes occurred in genes encoding key metabolic enzymes and stress response proteins associated with the TCA cycle (Fum: 18.6-fold, Aco: 17.1-fold, Oxo: 9.0-fold), carbon and energy metabolism (Tdh2: 28.0-fold, Erg2: 2.0-fold), electron transport chain (Gst: 10.6-fold), molecular chaperones (Hsp104: 26.9-fold, Hsp70: 13.0-fold, Sgt2: 10.0-fold), and transcriptional activators. Our findings indicate that the enhanced acetic acid tolerance of P. kudriavzevii PkAC-9 primarily depends on the coordinated upregulation of genes involved in energy metabolism, cellular detoxification mechanisms, and protein quality control systems through heat shock and transcriptional activator proteins.
Keywords: ethanol production; lignocellulosic biomass; real-time PCR; thermotolerant yeast.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Adaptive laboratory evolution under acetic acid stress enhances the multistress tolerance and ethanol production efficiency of Pichia kudriavzevii from lignocellulosic biomass.Sci Rep. 2023 Nov 28;13(1):21000. doi: 10.1038/s41598-023-48408-7. Sci Rep. 2023. PMID: 38017261 Free PMC article.
-
The potential of the newly isolated thermotolerant yeast Pichia kudriavzevii RZ8-1 for high-temperature ethanol production.Braz J Microbiol. 2018 Apr-Jun;49(2):378-391. doi: 10.1016/j.bjm.2017.09.002. Epub 2017 Nov 4. Braz J Microbiol. 2018. PMID: 29154013 Free PMC article.
-
Physiological responses contributing to multiple stress tolerance in Pichia kudriavzevii with potential enhancement for ethanol fermentation.J Biosci Bioeng. 2024 Oct;138(4):314-323. doi: 10.1016/j.jbiosc.2024.07.012. Epub 2024 Aug 3. J Biosci Bioeng. 2024. PMID: 39098474
-
Acetic acid stress in budding yeast: From molecular mechanisms to applications.Yeast. 2021 Jul;38(7):391-400. doi: 10.1002/yea.3651. Epub 2021 May 27. Yeast. 2021. PMID: 34000094 Free PMC article. Review.
-
Xylose utilization in Saccharomyces cerevisiae during conversion of hydrothermally pretreated lignocellulosic biomass to ethanol.Appl Microbiol Biotechnol. 2020 Apr;104(8):3245-3252. doi: 10.1007/s00253-020-10427-z. Epub 2020 Feb 19. Appl Microbiol Biotechnol. 2020. PMID: 32076775 Review.
References
-
- Sritrakul N., Nitisinprasert S., Keawsompong S. Evaluation of dilute acid pretreatment for bioethanol fermentation from sugarcane bagasse pith. Agri. Nat. Resour. 2017;51:512–519. doi: 10.1016/j.anres.2017.12.006. - DOI
-
- Cunha J.T., Costa C.E., Ferraz L., Romaní A., Johansson B., Sá-Correia I., Domingues L. HAA1 and PRS3 overexpression boosts yeast tolerance towards acetic acid improving xylose or glucose consumption: Unravelling the underlying mechanisms. Appl. Microbiol. Biotechnol. 2018;102:4589–4600. doi: 10.1007/s00253-018-8955-z. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

