Intra-Leaf Variability of Incubation Period Sheds New Light on the Lifestyle of Cercospora beticola in Sugar Beets
- PMID: 40137249
- PMCID: PMC11943282
- DOI: 10.3390/jof11030211
Intra-Leaf Variability of Incubation Period Sheds New Light on the Lifestyle of Cercospora beticola in Sugar Beets
Abstract
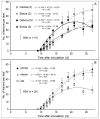
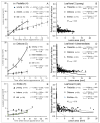
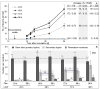
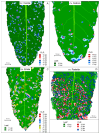
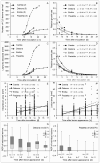
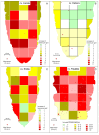
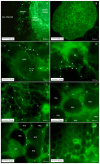
The length of incubation period, i.e., the time between first contact of host and pathogen and the appearance of symptoms, varies among diseases and depends on environmental conditions. Cercospora beticola is the most important fungal pathogen in sugar beet production worldwide, as Cercospora leaf spot (CLS) reduces the leaf area contributing to yield formation. Using sugar beet cultivars differing in CLS resistance, a single infection period of C. beticola resulted in minor differences in the incubation period among host genotypes and among individual plants of cultivars, greater differences among leaves within plants, and substantial variation within individual leaves. Under greenhouse conditions not suitable for secondary infections, the first CLS lesions appeared 10 days after inoculation; however, the number of leaf spots and CLS severity further increased significantly for another 7 to 17 days. A geographic information system approach enabled the tracking of colony appearance and growth of all CLSs on inoculated leaves for up to 27 days. Asymptomatic colonization of leaves was associated with thick hyphae which switched to thin hyphae or melanization after lesion appearance. The lifestyle of C. beticola-intercellular tissue colonization, triggering of necrotic host reaction-is discussed considering the experimental results as well as literature resources.
Keywords: asymptomatic colonization; hemibiotrophy; hyphal dimorphism; necrotrophy; toxin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Sensory assessment of Cercospora beticola sporulation for phenotyping the partial disease resistance of sugar beet genotypes.Plant Methods. 2019 Nov 16;15:133. doi: 10.1186/s13007-019-0521-x. eCollection 2019. Plant Methods. 2019. PMID: 31788018 Free PMC article.
-
Cercospora beticola: The intoxicating lifestyle of the leaf spot pathogen of sugar beet.Mol Plant Pathol. 2020 Aug;21(8):1020-1041. doi: 10.1111/mpp.12962. Mol Plant Pathol. 2020. PMID: 32681599 Free PMC article.
-
First Report of Cercospora beticola Causing a Leaf Spot Disease on Acanthus mollis in California.Plant Dis. 2011 Feb;95(2):224. doi: 10.1094/PDIS-10-10-0700. Plant Dis. 2011. PMID: 30743438
-
An in-field heat treatment to reduce Cercospora beticola survival in plant residue and improve Cercospora leaf spot management in sugarbeet.Front Plant Sci. 2023 May 9;14:1100595. doi: 10.3389/fpls.2023.1100595. eCollection 2023. Front Plant Sci. 2023. PMID: 37229110 Free PMC article.
-
Cercospora leaf spot disease of sugar beet.Plant Signal Behav. 2023 Dec 31;18(1):2214765. doi: 10.1080/15592324.2023.2214765. Plant Signal Behav. 2023. PMID: 37209061 Free PMC article. Review.
References
-
- Shane W., Teng P. Impact of Cercospora leaf spot on root weight, sugar yield, and purity of Beta vulgaris. Plant Dis. 1992;76:812–820. doi: 10.1094/PD-76-0812. - DOI
LinkOut - more resources
Full Text Sources

