The Hidden Fortress: A Comprehensive Review of Fungal Biofilms with Emphasis on Cryptococcus neoformans
- PMID: 40137272
- PMCID: PMC11943451
- DOI: 10.3390/jof11030236
The Hidden Fortress: A Comprehensive Review of Fungal Biofilms with Emphasis on Cryptococcus neoformans
Abstract
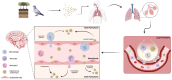
Biofilms are structurally organized communities of microorganisms that adhere to a variety of surfaces. These communities produce protective matrices consisting of polymeric polysaccharides, proteins, nucleic acids, and/or lipids that promote shared resistance to various environmental threats, including chemical, antibiotic, and immune insults. While algal and bacterial biofilms are more apparent in the scientific zeitgeist, many fungal pathogens also form biofilms. These surprisingly common biofilms are morphologically distinct from the multicellular molds and mushrooms normally associated with fungi and are instead an assemblage of single-celled organisms. As a collection of yeast and filamentous cells cloaked in an extracellular matrix, fungal biofilms are an extreme threat to public health, especially in conjunction with surgical implants. The encapsulated yeast, Cryptococcus neoformans, is an opportunistic pathogen that causes both pulmonary and disseminated infections, particularly in immunocompromised individuals. However, there is an emerging trend of cryptococcosis among otherwise healthy individuals. C. neoformans forms biofilms in diverse environments, including within human hosts. Notably, biofilm association correlates with increased expression of multiple virulence factors and increased resistance to both host defenses and antifungal treatments. Thus, it is crucial to develop novel strategies to combat fungal biofilms. In this review, we discuss the development and treatment of fungal biofilms, with a particular focus on C. neoformans.
Keywords: Aspergillus; Candida; Coccidioides; Cryptococcus neoformans; Fusarium; Trichosporon; biofilm; central nervous system; cryptococcosis; extracellular matrix; fungus; meningoencephalitis; polysaccharide capsule; titan cell; virulence factors.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
-
- Liu S., Le Mauff F., Sheppard D.C., Zhang S. Filamentous fungal biofilms: Conserved and unique aspects of extracellular matrix composition, mechanisms of drug resistance and regulatory networks in Aspergillus fumigatus. NPJ Biofilms Microbiomes. 2022;8:83. doi: 10.1038/s41522-022-00347-3. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

