Cu-MOF-Decorated 3D-Printed Scaffolds for Infection Control and Bone Regeneration
- PMID: 40137362
- PMCID: PMC11942848
- DOI: 10.3390/jfb16030083
Cu-MOF-Decorated 3D-Printed Scaffolds for Infection Control and Bone Regeneration
Abstract
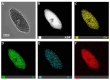
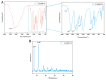
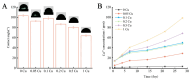
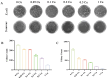
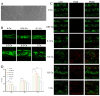
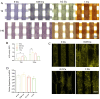
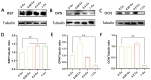
Infection control and bone regeneration remain critical challenges in bone defect treatment. We developed a 3D-printed scaffold incorporating copper-based metal-organic framework-74 (Cu-MOF-74) within a polycaprolactone/hydroxyapatite composite. The synthesized Cu-MOF-74 exhibited a well-defined crystalline structure and rod-like morphology, as confirmed by TEM, EDS, FTIR, and XRD analyses. The scaffolds exhibited hierarchical pores (100-200 μm) and demonstrated tunable hydrophilicity, as evidenced by the water contact angles decreasing from 103.3 ± 2.02° (0% Cu-MOF-74) to 63.60 ± 1.93° (1% Cu-MOF-74). A biphasic Cu2+ release profile was observed from the scaffolds, reaching cumulative concentrations of 98.97 ± 3.10 ppm by day 28. Antimicrobial assays showed concentration-dependent efficacy, with 1% Cu-MOF-74 scaffolds achieving 90.07 ± 1.94% and 80.03 ± 2.17% inhibition against Staphylococcus aureus and Escherichia coli, respectively. Biocompatibility assessments using bone marrow-derived mesenchymal stem cells revealed enhanced cell proliferation at Cu-MOF-74 concentrations ≤ 0.2%, while concentrations ≥ 0.5% induced cytotoxicity. Osteogenic differentiation studies highlighted elevated alkaline phosphatase activity and mineralization in scaffolds with 0.05-0.2% Cu-MOF-74 scaffolds, particularly at 0.05% Cu-MOF-74 scaffolds, which exhibited the highest calcium deposition and upregulation of bone sialoprotein and osteopontin expression. These findings demonstrate the dual functional efficacy of Cu-MOF-74/PCL/HAp scaffolds in promoting both infection control and bone regeneration. These optimized Cu-MOF-74 concentrations (0.05-0.2%) effectively balance antimicrobial and osteogenic properties, presenting a promising strategy for bone defect repair in clinical applications.
Keywords: 3D printing scaffold; antibacterial properties; bone regeneration; copper; metal–organic frameworks; osteoinductive potential.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

