Tracking Migraine Symptoms: A Longitudinal Comparison of Smartphone-Based Headache Diaries and Clinical Interviews
- PMID: 40137454
- PMCID: PMC11944553
- DOI: 10.3390/neurolint17030033
Tracking Migraine Symptoms: A Longitudinal Comparison of Smartphone-Based Headache Diaries and Clinical Interviews
Abstract
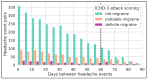
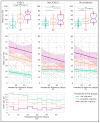
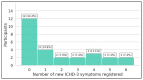
Background/Objectives: By leveraging the capabilities of a smartphone-based headache diary, the objective of this study was to determine the amount of agreement between migraine-associated symptomatology during headache events and the symptoms documented during clinician-led intake interviews. Methods: This was a 90-day longitudinal, smartphone-based headache calendar study for participants diagnosed with migraine. Registered headache events were labeled as "definite migraine", "probable migraine", and "not migraine" in accordance with the International Classification of Headache Disorders, Third Edition (ICHD-3) criteria. Symptoms' agreement with clinician-led intake interviews (agreement percentages and kappa coefficients), symptoms' similarity between headache events within users (percentage), and amount of newly registered ICHD-3 symptoms per participant were calculated. Results: Twenty-seven participants provided 505 headache events eligible for analysis. The median agreement between recorded headache event symptomatology and clinician-led intake interview phenotyping ranged between 40% (for events fulfilling "not migraine" criteria) and 55.5% ("definite migraine") (p < 0.001). Higher intraparticipant headache event pair similarity was observed for "definite migraine" pairs (p < 0.01), along with a decreasing trend in similarity as the attack-pair headache distance increases. Over half of the participants registered at least one new ICHD-3 symptom during the study. Conclusions: Electronic diary registrations show substantial longitudinal variability in intrapersonal headache symptomatology, with the similarity of headache events declining over time. The registration of a new ICHD-3 symptom was the rule rather than the exception.
Keywords: diary; headache; mHealth; migraine; prospective; smartphone.
Conflict of interest statement
Nicolas Vandenbussche has received travel grants and consulting fees from Novartis AG, Lundbeck, TEVA Pharmaceuticals Industries Ltd., AbbVie/Allergan, and Pfizer Inc. Koen Paemeleire has received personal compensation from AbbVie/Allergan, Amgen/Novartis AG, Eli Lilly and Company, Lundbeck, Pfizer, Teva Pharmaceuticals Industries Ltd., and Man&Science for consulting, serving on a scientific advisory board, and/or speaking; he is/was a clinical trial investigator for Almirall (almotriptan), Amgen/Novartis AG (erenumab), Eli Lilly and Company (galcanezumab, lasmiditan), Lundbeck (eptinezumab), and Autonomic Technologies Inc. (sphenopalatine ganglion stimulation). Jonas Van Der Donckt, Mathias De Brouwer, Bram Steenwinckel, Marija Stojchevska, Femke Ongenae, and Sofie Van Hoecke report no conflicts of interest.
Figures

Similar articles
-
A critical appraisal of the International Classification of Headache Disorders migraine diagnostic criteria based on a retrospective multicenter cross-sectional headache registry study in youth.Headache. 2024 Nov-Dec;64(10):1217-1229. doi: 10.1111/head.14858. Epub 2024 Oct 27. Headache. 2024. PMID: 39463026
-
A clinical interview versus prospective headache diaries in the diagnosis of menstrual migraine without aura.Cephalalgia. 2015 Apr;35(5):410-6. doi: 10.1177/0333102414545891. Epub 2014 Aug 20. Cephalalgia. 2015. PMID: 25143553
-
Circadian variations in the clinical presentation of headaches among migraineurs: A study using a smartphone headache diary.Chronobiol Int. 2018 Apr;35(4):546-554. doi: 10.1080/07420528.2017.1420076. Epub 2017 Dec 28. Chronobiol Int. 2018. PMID: 29283309
-
Migraine without aura.Handb Clin Neurol. 2023;198:151-167. doi: 10.1016/B978-0-12-823356-6.00007-X. Handb Clin Neurol. 2023. PMID: 38043959 Review.
-
Contributions of epidemiology to our understanding of migraine.Headache. 2013 Feb;53(2):230-46. doi: 10.1111/head.12038. Headache. 2013. PMID: 23432441 Review.
References
-
- Stovner L.J., Nichols E., Steiner T.J., Abd-Allah F., Abdelalim A., Al-Raddadi R.M., Ansha M.G., Barac A., Bensenor I.M., Doan L.P., et al. Global, Regional, and National Burden of Migraine and Tension-Type Headache, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:954–976. doi: 10.1016/S1474-4422(18)30322-3. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

