Exposure to Nanoplastics During Pregnancy Induces Brown Adipose Tissue Whitening in Male Offspring
- PMID: 40137498
- PMCID: PMC11945425
- DOI: 10.3390/toxics13030171
Exposure to Nanoplastics During Pregnancy Induces Brown Adipose Tissue Whitening in Male Offspring
Abstract
Background: Polystyrene nanoplastics (PSNPs) have been recognized as emerging environmental pollutants with potential health impacts, particularly on metabolic disorders. However, the mechanism by which gestational exposure to PSNPs induces obesity in offspring remains unclear. This study, focused on the whitening of brown adipose tissue (BAT), aims to elucidate the fundamental mechanisms by which prenatal exposure to PSNPs promotes obesity development in mouse offspring.
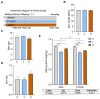
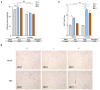
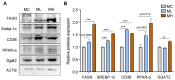
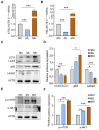
Methods and results: Pregnant dams were subjected to various doses of PSNPs (0 µg/µL, 0.5 µg/µL, and 1 µg/µL), and their offspring were analyzed for alterations in body weight, adipose tissue morphology, thermogenesis, adipogenesis, and lipophagy. The findings revealed a notable reduction in birth weight and an increase in white adipocyte size in adult offspring mice. Notably, adult male mice exhibited BAT whitening, correlated with a negative dose-dependent downregulation of UCP1 expression, indicating thermogenesis dysfunction. Further investigation revealed augmented lipogenesis evidenced by the upregulation of FASN, SREBP-1c, CD36, and DGAT2 expression, coupled with the inhibition of lipophagy, indicated by elevated levels of mTOR, AKT, and p62 proteins and reduced levels of LC3II/LCI and Lamp2 proteins in male offspring.
Conclusions: These findings indicate that gestational PSNP exposure plays a role in the development of obesity in offspring through the whitening of brown adipose tissue, which is triggered by lipogenesis and lipophagy inhibition, providing a novel insight into the metabolic risks associated with gestational PSNPs exposure.
Keywords: brown adipose tissue whitening; lipogenesis; lipophagy; obesity; polystyrene nanoplastics; pregnancy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Resveratrol supplementation of high-fat diet-fed pregnant mice promotes brown and beige adipocyte development and prevents obesity in male offspring.J Physiol. 2017 Mar 1;595(5):1547-1562. doi: 10.1113/JP273478. Epub 2017 Jan 24. J Physiol. 2017. PMID: 27891610 Free PMC article.
-
Maternal nicotine exposure during pregnancy and lactation induces brown adipose tissue whitening in female offspring.Toxicol Appl Pharmacol. 2020 Dec 15;409:115298. doi: 10.1016/j.taap.2020.115298. Epub 2020 Oct 19. Toxicol Appl Pharmacol. 2020. PMID: 33091441
-
Dietary succinate supplementation to maternal mice improves fetal brown adipose tissue development and thermogenesis of female offspring.J Nutr Biochem. 2022 Feb;100:108908. doi: 10.1016/j.jnutbio.2021.108908. Epub 2021 Nov 18. J Nutr Biochem. 2022. PMID: 34801687 Free PMC article.
-
An insight into brown/beige adipose tissue whitening, a metabolic complication of obesity with the multifactorial origin.Front Endocrinol (Lausanne). 2023 Feb 16;14:1114767. doi: 10.3389/fendo.2023.1114767. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36875450 Free PMC article. Review.
-
The Whitening of Brown Fat and Its Implications for Weight Management in Obesity.Curr Obes Rep. 2015 Jun;4(2):224-9. doi: 10.1007/s13679-015-0157-8. Curr Obes Rep. 2015. PMID: 26627217 Review.
References
Grants and funding
- 82173554/National Natural Science Foundation of China
- 22KJB330003/Natural Science Research of Jiangsu Higher Education Institutions
- 2023103041077/Nantong University College Students' Innovation and Entrepreneurship Training Program
- 2024200/Nantong University College Students' Innovation and Entrepreneurship Training Program
LinkOut - more resources
Full Text Sources
Miscellaneous

