The Evolution of In Vitro Toxicity Assessment Methods for Oral Cavity Tissues-From 2D Cell Cultures to Organ-on-a-Chip
- PMID: 40137522
- PMCID: PMC11946525
- DOI: 10.3390/toxics13030195
The Evolution of In Vitro Toxicity Assessment Methods for Oral Cavity Tissues-From 2D Cell Cultures to Organ-on-a-Chip
Abstract
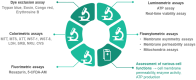
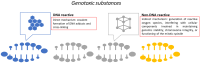
Since the oral cavity comes into contact with several xenobiotics (dental materials, oral hygiene formulations, drugs, or tobacco products), it is one major site for toxicity manifestation. Multiple parameters are assessed during toxicity testing (cell viability and proliferation, apoptosis, morphological changes, genotoxicity, oxidative stress, and inflammatory response). Due to the complexity of the oral cavity environment, researchers have made great efforts to design better in vitro models that mimic natural human anatomic and functional features. The present review describes the in vitro methods currently used to investigate the toxic potential of various agents on oral cavity tissues and their evolution from simple 2D cell culture systems to complex organ-a-chip designs.
Keywords: 3D oral tissue models; cytotoxicity; genotoxicity; oxidative stress; tooth-on-a-chip.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
A multi-organ-chip co-culture of liver and testis equivalents: a first step toward a systemic male reprotoxicity model.Hum Reprod. 2020 May 1;35(5):1029-1044. doi: 10.1093/humrep/deaa057. Hum Reprod. 2020. PMID: 32390056
-
3D Cell Cultures: Evolution of an Ancient Tool for New Applications.Front Physiol. 2022 Jul 22;13:836480. doi: 10.3389/fphys.2022.836480. eCollection 2022. Front Physiol. 2022. PMID: 35936888 Free PMC article. Review.
-
Dietary glycation compounds - implications for human health.Crit Rev Toxicol. 2024 Sep;54(8):485-617. doi: 10.1080/10408444.2024.2362985. Epub 2024 Aug 16. Crit Rev Toxicol. 2024. PMID: 39150724
-
A review of co-culture models to study the oral microenvironment and disease.J Oral Microbiol. 2020 Jun 4;12(1):1773122. doi: 10.1080/20002297.2020.1773122. J Oral Microbiol. 2020. PMID: 32922679 Free PMC article. Review.
-
3D organotypic HepaRG cultures as in vitro model for acute and repeated dose toxicity studies.Toxicol In Vitro. 2014 Feb;28(1):104-12. doi: 10.1016/j.tiv.2013.06.024. Epub 2013 Jul 9. Toxicol In Vitro. 2014. PMID: 23850736
References
-
- Biological Evaluation of Medical Devices Part 5: Tests for in Vitro Cytotoxicity. International Organization for Standardization; Geneva, Switzerland: 2009.
Publication types
LinkOut - more resources
Full Text Sources

